内生真菌的抗炎二聚体四羟基蒽酮
IF 3.6
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 5
摘要
从内生真菌Muyocopron laterale中分离到12个新的二聚体四羟基蒽酮,muyocoxanthones A-L(1-12)。根据核磁共振和hremsims资料的解释对其结构进行了表征。通过ECD谱数据和单晶x射线衍射分析,明确了1-10和12的绝对构型。化合物2、6和11对lps诱导的RAW 264.7细胞产生一氧化氮(NO)具有抑制作用,IC50值分别为5.2、1.3和5.1 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
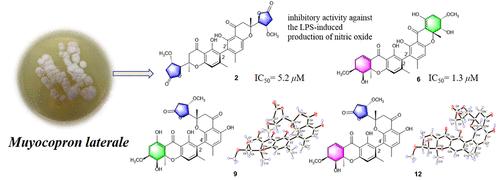
Anti-inflammatory Dimeric Tetrahydroxanthones from an Endophytic Muyocopron laterale
Twelve new dimeric tetrahydroxanthones, muyocoxanthones A–L (1–12), were isolated from the endophytic fungus, Muyocopron laterale. Their structures were characterized on the basis of the interpretation of NMR and HRESIMS data. The absolute configurations of 1–10 and 12 were unambiguously determined by ECD spectrum data and single-crystal X-ray diffraction analysis. Compounds 2, 6, and 11 showed inhibitory activity against the LPS-induced production of nitric oxide (NO) in RAW 264.7 cells with IC50 values of 5.2, 1.3, and 5.1 μM, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


