花粉 PCP-B 肽开启了柱头肽受体激酶授粉的门控机制
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 75
摘要
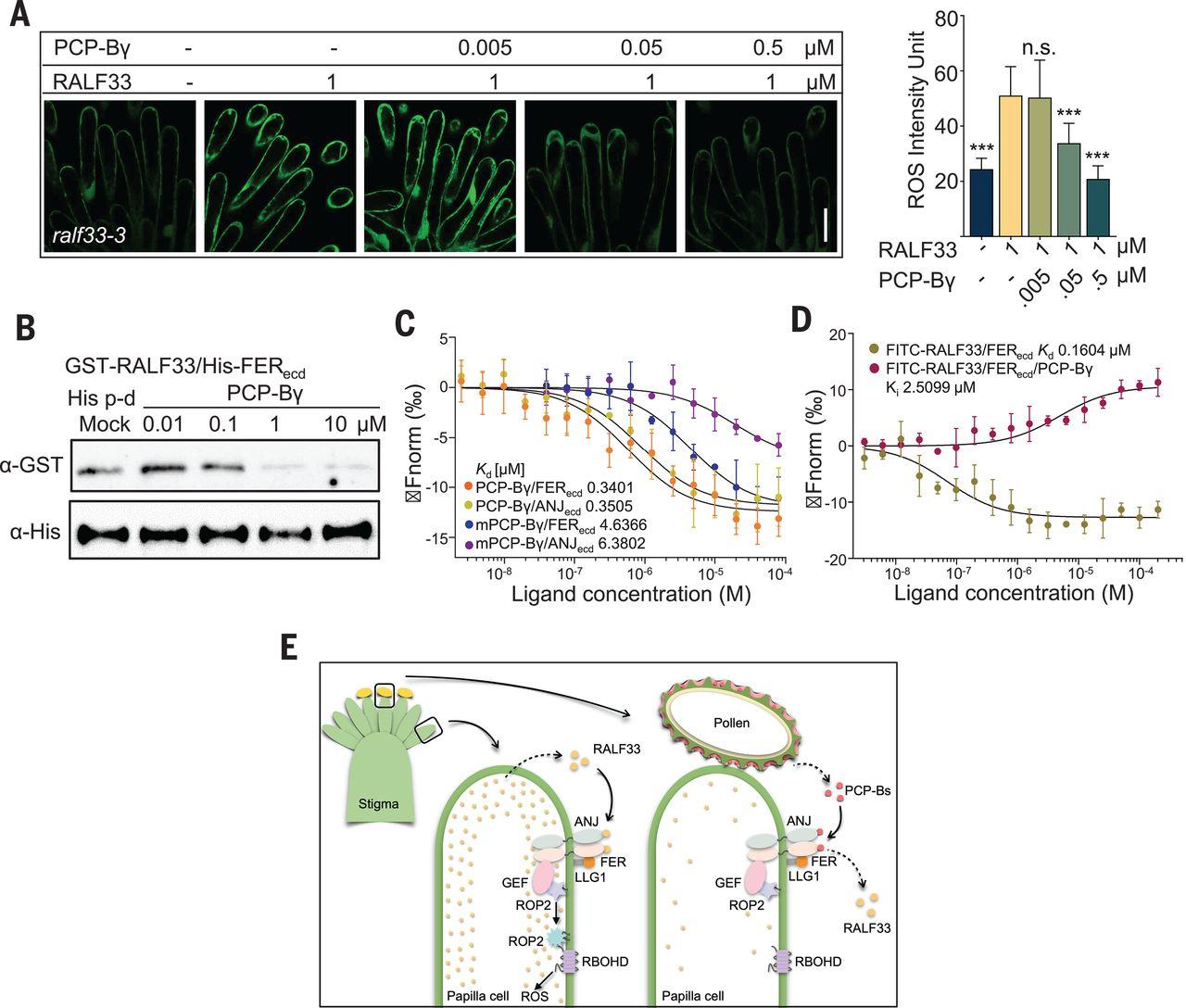
被子植物的有性生殖依赖于花粉和雌蕊之间的精确通信。这些沟通的分子机制仍然难以捉摸。我们发现,在拟南芥中,柱头守门人 ANJEA-FERONIA (ANJ-FER)受体激酶复合物会感知 RAPID ALKALINIZATION FACTOR 肽 RALF23 和 RALF33,从而诱导柱头乳头产生活性氧(ROS),而授粉会减少柱头的 ROS,使花粉水合。授粉时,柱头衣壳蛋白 B 级肽(PCP-Bs)与 RALF23/33 竞争结合到 ANJ-FER 复合物,导致柱头 ROS 减少,从而促进花粉水合。我们的研究结果阐明了一种分子门控机制,在这种机制中,来自花粉的不同肽类与柱头肽竞争与柱头受体激酶复合物的相互作用,从而使花粉水合和发芽。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pollen PCP-B peptides unlock a stigma peptide–receptor kinase gating mechanism for pollination
Sexual reproduction in angiosperms relies on precise communications between the pollen and pistil. The molecular mechanisms underlying these communications remain elusive. We established that in Arabidopsis, a stigmatic gatekeeper, the ANJEA–FERONIA (ANJ–FER) receptor kinase complex, perceives the RAPID ALKALINIZATION FACTOR peptides RALF23 and RALF33 to induce reactive oxygen species (ROS) production in the stigma papillae, whereas pollination reduces stigmatic ROS, allowing pollen hydration. Upon pollination, the POLLEN COAT PROTEIN B-class peptides (PCP-Bs) compete with RALF23/33 for binding to the ANJ–FER complex, leading to a decline of stigmatic ROS that facilitates pollen hydration. Our results elucidate a molecular gating mechanism in which distinct peptide classes from pollen compete with stigma peptides for interaction with a stigmatic receptor kinase complex, allowing the pollen to hydrate and germinate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


