白藜芦醇及其糖基化和酰化衍生物的构效关系及其α-葡萄糖苷酶抑制活性的研究
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
白藜芦醇具有良好的α-葡萄糖苷酶抑制活性,广泛存在于食品中。然而,白藜芦醇糖基化和酰化对其α-葡萄糖苷酶抑制活性影响的深入研究有限。本文研究了白藜芦醇、白藜芦醇-4′- o -葡萄糖苷、白藜芦醇4′- o -(6′- o -没食子酰)-葡萄糖苷和白藜芦醇4′- o -(2′- o -没食子酰)-葡萄糖苷对α-葡萄糖苷酶的构效关系。值得注意的是,C-4 '糖基化增强了活性,随后的没食子酰酰化进一步提高了活性,其中C-6的酰化“略优于C-2”。光谱分析证实了酶在结合时的构象变化。分子对接表明,C-4′糖基化导致与α-葡萄糖苷酶形成额外的氢键,随后的酰化进一步增加了它们的数量。分子动力学模拟表明,白藜芦醇4′- o -(6”- o -没食子酰)-葡萄糖苷由于其空间位阻比白藜芦醇4′- o -(2”- o -没食子酰)-葡萄糖苷低,能更深入地渗透到α-葡萄糖苷酶的活性位点口袋中,从而使其与α-葡萄糖苷的结合更加稳定。研究结果可为白藜芦醇及其衍生物在功能性食品中的应用提供理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
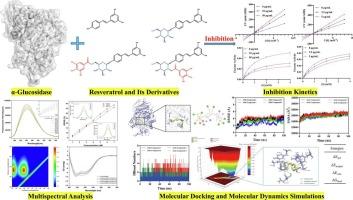
Investigation of the structure-activity relationship of resveratrol and its glycosylated and acylated derivatives in relation to their α-glucosidase inhibitory activities
Resveratrol with excellent α-glucosidase inhibitory activity is widely distributed in foods. However, in-depth research on the effects of glycosylation and acylation of resveratrol on its α-glucosidase inhibitory activity is limited. In the present study, we investigated the structure-activity relationship of resveratrol, resveratrol-4’-O-glucoside, resveratrol 4’-O-(6′′-O-galloyl)-glucoside and resveratrol 4’-O-(2′′-O-galloyl)-glucoside against α-glucosidase. Notably, C-4′ glycosylation enhanced potency, and subsequent galloyl acylation further improved activity with acylation at C-6′′ slightly outperforming that at C-2′′. Spectroscopic analyses confirmed conformational changes in the enzyme upon binding. Molecular docking showed C-4′ glycosylation led to the formation of additional hydrogen bonds with α-glucosidase, and subsequent acylation further increased their number. Molecular dynamics simulations showed resveratrol 4’-O-(6′′-O-galloyl)-glucoside, owing to its lower steric hindrance than resveratrol 4’-O-(2′′-O-galloyl)-glucoside, penetrates more deeply into the active-site pocket of α-glucosidase, resulting in a more stable binding. These findings may provide theoretical basis for the development of resveratrol and its derivatives in functional foods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


