能同时降解黄曲霉毒素B1和玉米赤霉烯酮的土曲霉HNGD-TM15二肽基肽酶III的两亲性和热稳定性修饰
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
黄曲霉毒素B1 (AFB1)和玉米赤霉烯酮(ZEN)是严重威胁食品安全和人类健康的重要真菌毒素。本研究从土曲霉HNGD-TM15中分离出一种二肽基肽酶III (AsDPP III),该酶能降解98.25%的AFB1 (5.0 μg/mL)和96.68%的ZEN (10 μg/mL)。此外,一个自组装的两亲肽S1被融合到AsDPP III的n端,形成两亲酶S1-AsDPP III,该酶对植物油中AFB1和ZEN的降解率分别为83.36%和70.08%。此外,S1-AsDPP III表现出增强的热稳定性,其在80°C时的半衰期延长了1.85倍,并且在7周后具有优异的储存稳定性。此外,降解产物AFB1鉴定为AFQ1, ZEN鉴定为DHZEN。斑马鱼暴露试验显示,与母体真菌毒素相比,降解产物的毒性明显降低。这些发现为开发用于食品和饲料的高效解毒酶提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
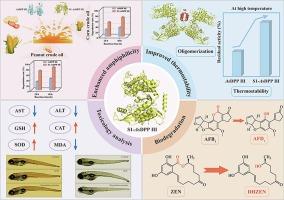
Amphiphilicity and thermostability modification of dipeptidyl peptidase III from aspergillus terreus HNGD-TM15 capable of simultaneously degrading aflatoxin B1 and zearalenone
Aflatoxin B1 (AFB1) and Zearalenone (ZEN) are significant mycotoxins that pose serious risks to food safety and human health. This study characterized a dipeptidyl peptidase III (AsDPP III) from Aspergillus terreus HNGD-TM15, which degraded 98.25 % of AFB1 (5.0 μg/mL) and 96.68 % of ZEN (10 μg/mL). Additionally, a self-assembling amphiphilic peptide S1 was fused to the N-terminus of AsDPP III, resulting in the amphiphilic enzyme S1-AsDPP III, which demonstrated 83.36 % and 70.08 % degradation rates for AFB1 and ZEN in vegetable oil, respectively. Moreover, S1-AsDPP III exhibits enhanced thermostability, with its half-life at 80 °C extended 1.85-fold, and superior storage stability after seven weeks. Furthermore, the degradation products were identified as AFQ1 for AFB1 and DHZEN for ZEN. Zebrafish exposure assays revealed that the degradation products exhibited markedly reduced toxicity compared to the parent mycotoxins. These findings provide a promising strategy for developing efficient detoxifying enzymes for food and feed applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


