氧化对蛇头肌原纤维蛋白乳化特性的影响:结构和界面变化的作用。
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
肌原纤维蛋白(myofibrar protein, MP)是肌肉类食品中重要的功能蛋白,对乳状液的稳定性有重要影响。然而,氧化对其界面行为的影响尚不清楚。本研究旨在阐明氧化修饰如何影响蛇头(Channa argus) MP的构象行为、其在油水界面的动态吸附以及相关的乳化性能。不同H2O2浓度(0、2、4、8、16 mM)对MP进行氧化处理。适度氧化(≤4 mM)增加了结构柔韧性和疏水性基团的暴露,从而提高了界面压力、扩散(Kdiff)、穿透(KP)和重排速率(KR)。由于界面膜致密,4mm组产生了最稳定的乳液,具有良好的流变性。相反,过度氧化(≥8 mM)促进聚集,埋藏疏水位点,降低界面活性,导致稳定性差。这些发现表明,控制氧化可以优化MP的功能,为开发蛋白质稳定性乳液体系提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
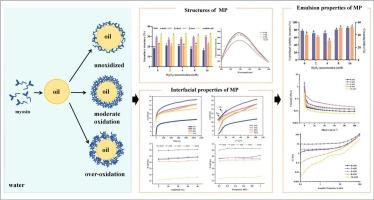
Effects of oxidation on the emulsion properties of snakehead (Channa argus) myofibrillar protein: Roles of structural and interfacial changes
Myofibrillar protein (MP), a key functional protein in muscle foods, strongly affects emulsion stability. However, the impact of oxidation on its interfacial behavior remained unclear. This study aimed to clarify how oxidative modification affects the conformational behavior of snakehead (Channa argus) MP, its dynamic adsorption at the oil-water interface, and the related emulsifying properties. MP was oxidized with different H2O2 concentrations (0, 2, 4, 8, 16 mM). Moderate oxidation (≤ 4 mM) increased structural flexibility and exposed hydrophobic groups, thereby enhancing interfacial pressure, diffusion (Kdiff), penetration (KP), and rearrangement rates (KR). The 4 mM group produced the most stable emulsion with favorable rheology due to a compact interfacial film. In contrast, excessive oxidation (≥ 8 mM) promoted aggregation, buried hydrophobic sites, and reduced interfacial activity, leading to poor stability. These findings demonstrated that controlled oxidation could optimize MP functionality, providing a theoretical basis for developing protein-stability emulsion systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


