线虫端粒酶RNA搭便车在种系上调基因的内含子上
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
端粒酶是一种核糖核蛋白复合物,可以延长端粒DNA,确保种系的不朽。在这项研究中,我们确定了秀丽隐杆线虫端粒酶RNA组分1 (terc-1),作为已知的第一个以内含子长链非编码RNA (lncRNA)表达的端粒酶RNA,嵌入在种系上调基因nmi -2的内含子中。terc-1经历剪接、聚腺苷化和核RNA外泌体依赖的成熟,由H/ACA小核仁核糖核蛋白稳定,从而选择H/ACA小核仁RNA (snoRNA)生物发生机制。terc-1的突变导致端粒逐渐缩短和后代不育。将nmy-2内含子人工移植到生殖系表达基因的内含子中,而不是非生殖系表达基因的内含子中,可以恢复生殖系的不朽性,这突出了基因组背景的重要性。我们的研究结果表明,线虫端粒酶RNA是一种类似于snorna的内含子lncRNA,它利用种系向上调节基因的内含子来确保物种生存。本文章由计算机程序翻译,如有差异,请以英文原文为准。
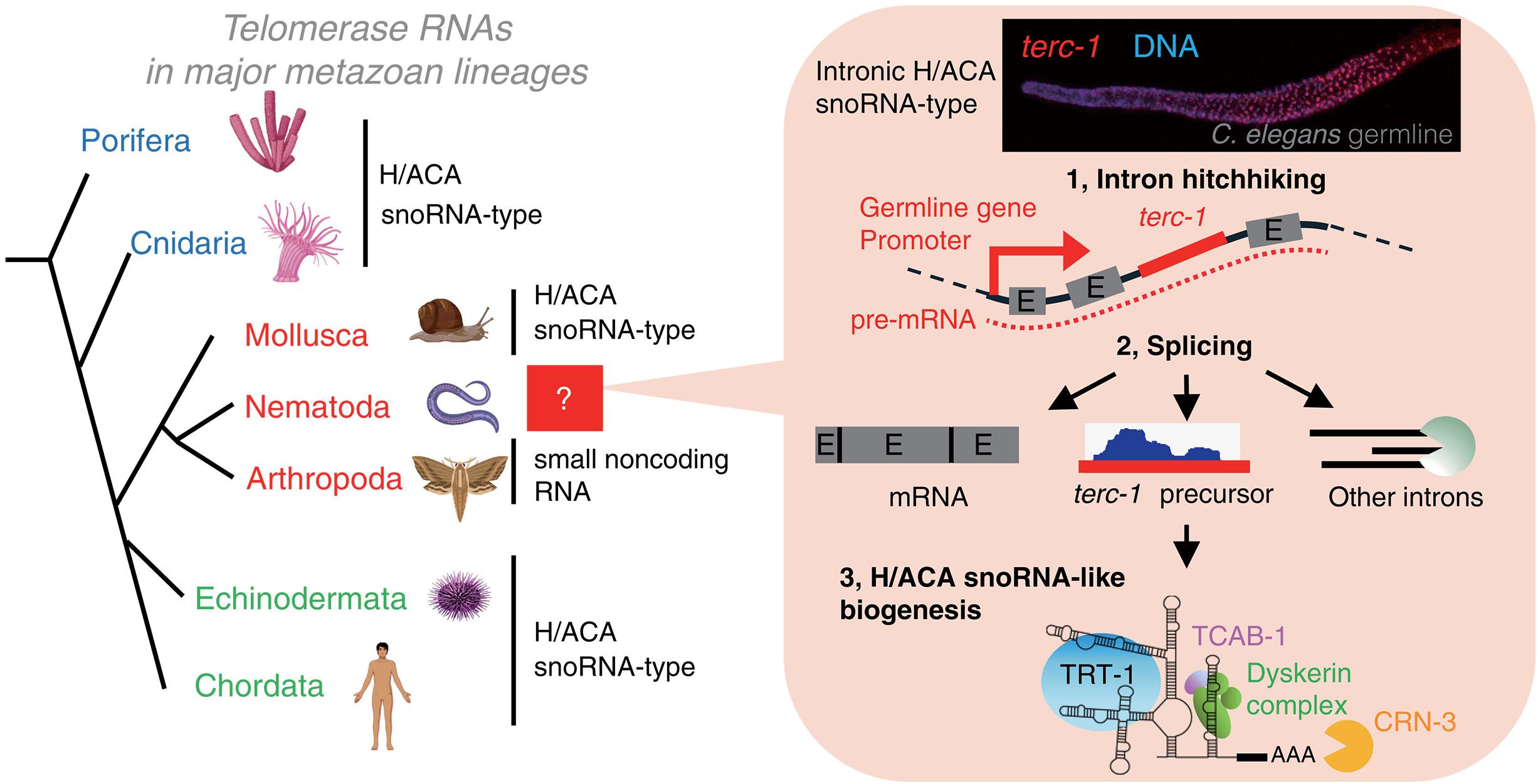
Nematode telomerase RNA hitchhikes on introns of germline–up-regulated genes
Telomerase is a ribonucleoprotein complex that elongates telomeric DNA, ensuring germline immortality. In this study, we identified the Caenorhabditis elegans telomerase RNA component 1 (terc-1), as the first known telomerase RNA expressed as an intronic long noncoding RNA (lncRNA), embedded in an intron of germline–up-regulated gene nmy-2. terc-1 undergoes splicing, polyadenylation, and nuclear RNA exosome–dependent maturation, stabilized by H/ACA small nucleolar ribonucleoproteins, thus co-opting the H/ACA small nucleolar RNA (snoRNA) biogenesis machinery. Mutations in terc-1 led to progressive telomere shortening and sterility in successive generations. Artificially transplanting the nmy-2 intron into the introns of germline-expressed genes but not non–germline-expressed genes restored germline immortality, highlighting the importance of genomic context. Our findings suggest that nematode telomerase RNA is a snoRNA-like intronic lncRNA that exploits the introns of germline–up-regulated genes to ensure species survival.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


