阳离子对魔芋葡甘露聚糖促凝胶作用对κ-卡拉胶相变的影响
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
通过将魔芋葡甘露聚糖(KGM)与κ-卡拉胶(κ-Car)共混,可以形成协同作用凝胶,其中柔韧的KGM链对κ-Car的相变具有熵驱动的促进作用,提高了其凝固温度(ΔTs)和熔化温度(ΔTm)。然而,KCl或CaCl2的加入逐渐削弱了这种促进作用,降低了KGM/κ-Car的ΔTs和ΔTm。在≥0.03 mol/L Ca2+浓度下,通过AFM和SEM观察,这些盐还引起了显著的微观结构变化,包括粗纤维结构和短致密束。SAXS结果表明,随着阳离子浓度的增加,结构域大小和相关长度都增加,表明网络的互联性增强,凝胶强度增强。凝胶硬度分别为729 g (K+)和774 g (Ca2+)。然而,过量的Ca2+导致过交联和凝胶变弱。这些发现强调了熵驱动的协同作用和离子交联之间的平衡,为设计具有定制协同性能的KGM/κ-Car凝胶提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
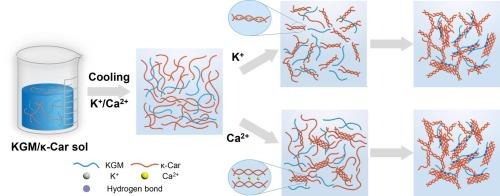
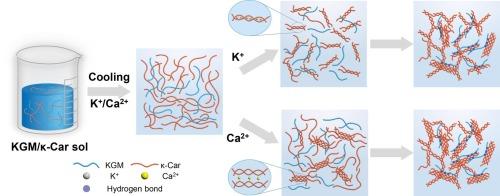
Influences of cations on the gelation-promoting effect of konjac glucomannan on the phase transition of κ-carrageenan
Synergistic interaction gels can be created by blending konjac glucomannan (KGM) and κ-carrageenan (κ-Car), where the flexible KGM chains suggest an entropy-driven promotion effect on κ-Car phase transition, increasing its setting temperature (ΔTs) and melting temperature (ΔTm). However, the addition of KCl or CaCl2 gradually weakened this promotion, reducing the ΔTs and ΔTm of the KGM/κ-Car. These salts also caused notable microstructural changes, including thick fibrillar structures and short dense bundles at ≥0.03 mol/L Ca2+, revealed by AFM and SEM. SAXS results showed that both domain size and correlation length increased with increasing cation concentration, indicating a more interconnected network and enhanced gel strength. Consequently, gel hardness increased to 729 g (K+) and 774 g (Ca2+). However, excessive Ca2+ led to over-crosslinking and weakened gels. These findings highlight the balance between entropy-driven synergy and ionic crosslinking, providing guidance for designing KGM/κ-Car gels with tailored synergistic properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


