电子穿梭机制驱动接近标记从临床样本揭示卵巢癌肿瘤标志物特征
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
在这项研究中,我们提出了一个革命性的接近标记平台MOF-HRP-Apt,它首次将电子中介集成到辣根过氧化物酶(HRP)催化的接近标记系统中。这种新策略将单个分子识别事件转化为多个共价标记事件,放大了空间信号,提高了肿瘤生物标志物PTK7的检测灵敏度。该平台利用具有氧化还原活性的铁基金属有机骨架(MOF)材料NH2-MIL-88B,其Fe2+/Fe3+氧化还原中心促进了电子转移到HRP的活性位点,显著提高了HRP对苯酚氧化的催化活性,加速了苯氧自由基的生成。这些自由基可以共价标记PTK7及其邻近蛋白中的酪氨酸残基,从而实现有效的空间定位。与传统的HRP-Apt策略相比,MOF-HRP-Apt平台在细胞模型中表现出更强的标记信号(增加1.74-3倍)和更高的信噪比(提高1.93 - 2.2倍)。即使在PTK7低表达、sirna介导的敲低或紫杉醇诱导的抑制等具有挑战性的条件下,它也能保持良好的性能。此外,在临床组织标本中,我们的平台成功地实现了PTK7在卵巢癌进展谱上的分层可视化,从正常组织到早期到晚期,证明了其在复杂生物环境中的卓越敏感性和适应性。通过结合靶向识别和信号放大,该策略提供了低丰度生物标志物的超灵敏检测。凭借其在早期癌症筛查、实时分子跟踪和个性化治疗开发方面的巨大潜力,我们的平台代表了分子诊断领域的重大飞跃。这项研究举例说明了电子介导的接近标记的变革力量,为推进精准医学和分子生物学提供了一条有前途的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
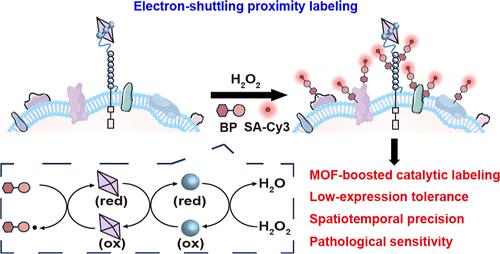
Electron-Shuttling Mechanisms Drive Proximity Labeling to Unveil Tumor Marker Characteristics in Ovarian Cancer from Clinical Samples
In this study, we presented a revolutionary proximity labeling platform, MOF-HRP-Apt, which for the first time integrated electron mediation into the horseradish peroxidase (HRP)-catalyzed proximity labeling system. This novel strategy enabled a single molecular recognition event into multiple covalent labeling events, amplifying spatial signals and enhancing detection sensitivity of the tumor biomarker PTK7. The platform utilized the redox-active iron-based metal–organic framework (MOF) material NH2-MIL-88B, whose Fe2+/Fe3+ redox center facilitated electron transfer to the active site of HRP, dramatically boosting HRP’s catalytic activity toward phenol oxidation and accelerating phenoxo radical generation. These radicals could covalently label tyrosine residues in PTK7 and its adjacent proteins to achieve efficient spatial localization. Compared to conventional HRP-Apt strategies, MOF-HRP-Apt platform exhibited significantly stronger labeling signals (1.74-3 folds increase) and improved signal-to-noise ratios (1.93−2.2 folds enhancement) in cellular models. It maintained robust performance even under challenging conditions of low PTK7 expression, siRNA-mediated knockdown, or paclitaxel-induced suppression. Moreover, in clinical tissue specimens, our platform successfully enabled stratified PTK7 visualization across the ovarian cancer progression spectrum─from normal tissue through early to advanced stages, demonstrating its exceptional sensitivity and adaptability in complex biological environments. By combining target-specific recognition with signal amplification, this strategy offered the ultrasensitive detection of low-abundance biomarkers. With its remarkable potential for early cancer screening, real-time molecular tracking, and personalized therapeutic development, our platform represents a significant leap forward in molecular diagnostics. This study exemplifies the transformative power of electron-mediated proximity labeling, offering a promising avenue for advancing precision medicine and molecular biology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


