一种19f标记的异硫氰酸酯衍生化剂用于环仲胺手性鉴别
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
由于环仲胺在药物开发中的广泛应用,快速检测和区分手性环仲胺的方法变得越来越重要。在这项研究中,我们成功地提出了一种衍生化策略,利用19f标记的异硫氰酸酯对各种环仲胺进行对映体分析,包括哌啶、吡啶、哌嗪和morpholines(28个例子)。由新型手性19f标记探针产生的可区分的化学位移允许在混合物中同时分化三对环仲胺。这种方法消除了底物共价修饰的繁琐纯化步骤,使这些手性n -杂环化合物的快速分析具有清晰的信号和高分辨率。此外,通过单晶x射线分析和DFT计算,首次验证了新型手性19F标记探针与分析物之间衍生相互作用的机制,该机制产生了一对具有不同19F核磁共振信号的非对映异构体硫脲。该技术为手性环二胺的分析提供了一种高效、灵敏和通用的解决方案,特别是在药物合成工艺的开发中具有重要的价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
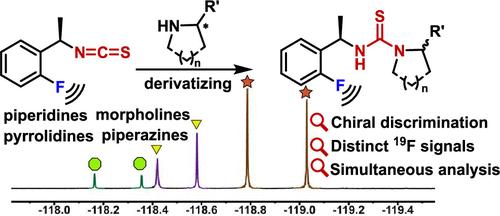
A 19F-Labeled Isothiocyanate Derivatizing Agent for the Chiral Discrimination of Cyclic Secondary Amines
Due to the widespread applications of cyclic secondary amines in drug development, rapid methods for detecting and distinguishing chiral cyclic secondary amines have become increasingly important. In this study, we successfully present a derivatizing strategy employing 19F-labeled isothiocyanate for the enantiomeric analysis of various cyclic secondary amines, including piperidines, pyrrolidines, piperazines, and morpholines (28 examples). The distinguishable chemical shift produced by the novel chiral 19F-labeled probe permits simultaneous differentiation of three pairs of cyclic secondary amines in a mixture. This approach eliminates the cumbersome purification steps of covalent modification of substrates, enabling rapid analysis of these chiral N-heterocyclic compounds with clear signals and high resolution. Besides, the mechanism of derivatizing interaction between a novel chiral 19F-labeled probe and the analytes was first verified by single-crystal X-ray analysis and DFT calculations, which generates a pair of diastereomeric thioureas with distinct 19F NMR signals. This technology provides an efficient, sensitive, and versatile solution for the analysis of chiral cyclic sec-amines, demonstrating significant value, particularly in the development of pharmaceutical synthesis processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


