裸鼹鼠体内cgas介导的机制增强DNA修复并延缓衰老
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
有效的DNA修复可能会使裸鼹鼠长寿。然而,它们是否具有独特的机制来优化DNA修复抑制子的功能尚不清楚。我们发现裸鼹鼠环鸟苷单磷酸-腺苷单磷酸合成酶(cGAS)在进化过程中通过改变4个氨基酸,在同源重组修复中缺乏人类或小鼠同源物的抑制功能。这些变化通过削弱trim41介导的泛素化和与分离酶P97的相互作用,使cGAS在DNA损伤时保留染色质的时间更长。延长cGAS的染色质结合增强了修复因子FANCI和RAD50之间的相互作用,促进了RAD50招募到损伤位点,从而增强了同源重组修复。此外,这四种氨基酸介导了cGAS抗细胞和组织衰老、延长寿命的功能。因此,操纵cGAS可能构成一种延长寿命的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
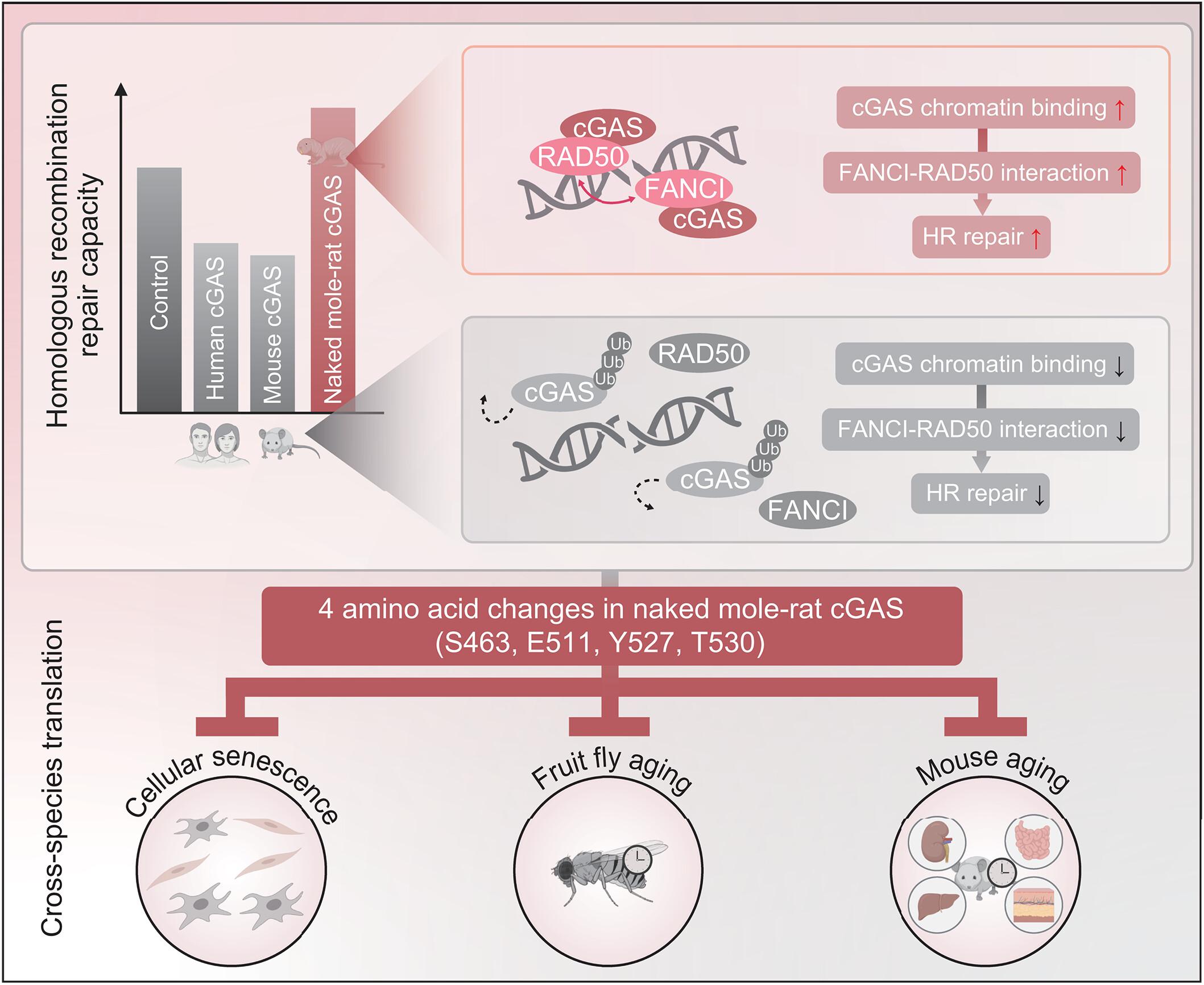
A cGAS-mediated mechanism in naked mole-rats potentiates DNA repair and delays aging
Efficient DNA repair might make possible the longevity of naked mole-rats. However, whether they have distinctive mechanisms to optimize functions of DNA repair suppressors is unclear. We find that naked mole-rat cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS) lacks the suppressive function of human or mouse homologs in homologous recombination repair through the alteration of four amino acids during evolution. The changes enable cGAS to retain chromatin longer upon DNA damage by weakening TRIM41-mediated ubiquitination and interaction with the segregase P97. Prolonged chromatin binding of cGAS enhanced the interaction between repair factors FANCI and RAD50 to facilitate RAD50 recruitment to damage sites, thereby potentiating homologous recombination repair. Moreover, the four amino acids mediate the function of cGAS in antagonizing cellular and tissue aging and extending life span. Manipulating cGAS might therefore constitute a mechanism for life-span extension.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


