13-Plex DeAla等压试剂用于高通量蛋白质组定量
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
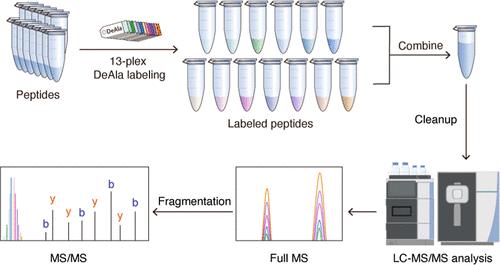
等压标记技术广泛应用于基于质谱的定量蛋白质组学,以实现多个样品的同时分析。然而,由于复杂的合成和昂贵的试剂,商用等压标签价格昂贵,限制了它们在大规模研究中的应用。在这里,我们介绍了一种新型的,具有成本效益的基于二乙基丙氨酸的等压试剂(DeAla),该试剂由二乙基丙氨酸和β-丙氨酸与n -羟基琥珀酰亚胺合成。DeAla标签具有几个优点,包括改进的肽片段,增强的蛋白质识别和有竞争力的价格。我们优化了标记效率和碰撞能量参数,结果表明,与N,N-二甲基亮氨酸(DiLeu)标记的肽相比,deala标记的肽产生更多的骨干断裂离子和更高的XCorr值。通过选择性地加入稳定同位素,我们在不增加结构复杂性的情况下将多路复用能力扩展到13路复用,实现了60k分辨率的Orbitrap MS/MS采集的基线分辨率。两种癌细胞系的比较蛋白质组学分析表明,DeAla标记优于DiLeu标记,并且在蛋白质和肽鉴定方面表现出与无标记方法相当的性能。此外,DeAla在动态范围内以最小的技术可变性提供准确和可重复的定量。总体而言,13-plex DeAla试剂是一种成本效益高、高性能的等压标记工具,可增强肽片段和蛋白质鉴定,同时确保高定量准确性,使其在复杂的定量蛋白质组学分析中具有价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
13-Plex DeAla Isobaric Reagents for High-Throughput Proteome Quantification
Isobaric labeling techniques are widely used in mass spectrometry-based quantitative proteomics to enable the simultaneous analysis of multiple samples. However, commercial isobaric tags are expensive due to complex synthesis and costly reagents, limiting their use in large-scale studies. Here, we introduce a novel, cost-effective diethylalanine-based isobaric reagent (DeAla), synthesized using diethylated alanine and β-alanine with N-hydroxysuccinimide. The DeAla tag offers several advantages, including improved peptide fragmentation, enhanced protein identification, and competitive pricing. We optimized labeling efficiency and collision energy parameters, demonstrating that DeAla-labeled peptides produce more backbone fragmentation ions and higher XCorr values compared to peptides labeled with N,N-dimethyl leucine (DiLeu) tags. By selectively incorporating stable isotopes, we expanded the multiplexing capacity to 13-plex without increasing structural complexity, achieving baseline resolution in Orbitrap MS/MS acquisition at 60k resolution. Comparative proteomic analyses of two cancer cell lines demonstrated that DeAla labeling outperformed DiLeu tags and showed comparable performance to label-free approaches in terms of protein and peptide identification. Additionally, DeAla provided accurate and reproducible quantification across a dynamic range with minimal technical variability. Overall, the 13-plex DeAla reagents are cost-effective, high-performance isobaric tagging tools that enhance peptide fragmentation and protein identification while ensuring high quantification accuracy, making them valuable for complex quantitative proteomic analyses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


