蛋白质组学差异分析的无监督机器学习
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
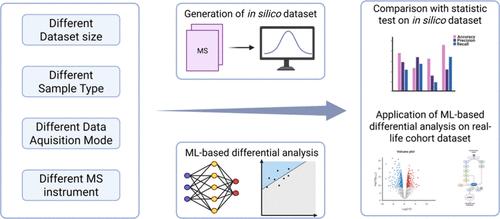
蛋白质组学中的差异分析是生物标志物发现和疾病机制阐明的关键,但传统的统计方法受到分布假设和经验折叠变化阈值依赖性的限制。本研究系统地评估了18种无监督异常检测机器学习(ML)算法,以对抗从蛋白质组学数据集中检测差异蛋白质的既定统计框架。使用来自实验数据的计算机模拟数据集,我们通过基于概率的转换实现了跨算法的可比性。结果表明,ML方法,特别是最小协方差行列式(MCD),在召回率、精密度和准确度方面优于统计检验,对样本间异质性具有较强的稳健性。真实世界蛋白质组学数据验证进一步证实,mcd鉴定的差异表达蛋白全面覆盖了典型途径,同时发现了新的肿瘤相关功能生物分子。这项工作建立了无监督机器学习方法,作为蛋白质组学差异分析中传统假设驱动的统计方法的强大替代品,为精准医学研究提供了更高的可靠性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unsupervised Machine Learning for Differential Analysis in Proteomics
Differential analysis in proteomics is pivotal for biomarker discovery and disease mechanism elucidation, yet traditional statistical methods are constrained by distributional assumptions and empirical fold change threshold dependencies. This study systematically evaluates 18 unsupervised anomaly detection machine learning (ML) algorithms against the established statistical frameworks for differential protein detection from proteomic data sets. Using in silico simulated data sets derived from experimental data, we enabled cross-algorithm comparability through a probability based transformation. Results demonstrated that ML methods, particularly the Minimum Covariance Determinant (MCD), outperformed statistical test in recall, precision, and accuracy, with superior robustness to intersample heterogeneity. Validation on real-world proteomic data further confirmed that the MCD-identified differentially expressed proteins comprehensively covered canonical pathways while uncovering novel tumor-associated functional biomolecules. This work establishes unsupervised ML methods as robust alternatives to traditional hypothesis-driven statistical approaches in proteomics differential analysis, offering enhanced reliability for precision medicine research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


