19F核磁共振引导手性分析和机器学习的绝对构型预测羧酸
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
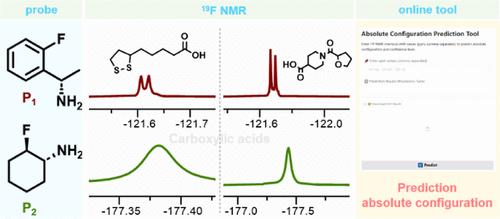
19f标记的分子探针在手性分析中具有显著优势,其立体结构在对映体识别中起着关键作用。快速准确的绝对构型分配对于制药领域和不对称合成尤为重要。本文介绍了两种19f标记探针作为CDAs的比较评价:芳香P1 ((S)-1-(2-氟苯基)乙胺)和脂肪P2(反式2-氟环己胺),重点关注它们对手性羧酸的对映体识别。结果表明P1优于P2,特别是在分析手性中心位于远离羧基的几个碳或具有多个手性中心的羧酸时。密度泛函理论计算阐明了潜在的机制,揭示了P1的高识别效率来自于非对映体之间较大的能量差以及其氟原子与被分析物质子之间形成的氢键。这些发现强调了探针-分析物相互作用在基于cda的对映体识别系统中实现高分辨率识别的重要性。此外,P1在测定不同品牌的布洛芬胶囊和片剂的ee值和分析中具有实际应用价值,突出了其在药品质量控制方面的潜力。此外,我们引入了一个新的在线平台,将p1引导的19F NMR方法与机器学习相结合,用于自动预测绝对构型。这项工作揭示了芳香和脂肪族探针在对构象识别方面的差异,同时引入了一个在线预测工具,有利于19F分子探针的设计和绝对构型的快速分配。本文章由计算机程序翻译,如有差异,请以英文原文为准。
19F NMR-Guided Chiral Analysis and Machine Learning for Absolute Configuration Prediction of Carboxylic Acids
19F-labeled molecular probes offer significant advantages in chiral analysis, with their stereostructures playing a critical role in enantiodiscrimination. Rapid and accurate assignment of absolute configurations is particularly important for the pharmaceutical field and asymmetric synthesis. This work presents a comparative evaluation of two 19F-labeled probes as CDAs: aromatic P1 ((S)-1-(2-fluorophenyl)ethylamine) and aliphatic P2 (trans-2-fluorocyclohexanamine), focusing on their enantiomeric recognition of chiral carboxylic acids. The results demonstrate that P1 outperformed P2, particularly in analyzing carboxylic acids with chiral centers positioned several carbons away from the carboxyl group or possessing multiple chiral centers. Density functional theory calculations elucidated the underlying mechanisms, revealing that P1’s great recognition efficiency arises from a larger energy difference between diastereomers and the formation of hydrogen bonds between its fluorine atom and the analytes’ protons. These findings emphasize the importance of probe–analyte interactions in CDA-based enantiorecognition systems for achieving high-resolution discrimination. Moreover, P1 exhibited practical applications in determining ee values and analyzing various brands of ibuprofen capsules and tablets, highlighting its potential for pharmaceutical quality control. Furthermore, we introduced a novel online platform combining a P1-guided 19F NMR approach with machine learning for automated prediction of absolute configurations. This work sheds light on the differences between aromatic and aliphatic probes in enantiorecognition, while introducing an online predictive tool, in favor of the design of 19F molecular probes and the rapid assignment of absolute configurations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


