将气溶胶酸度测量扩展到水限制状态:冷凝生长和比色法的耦合方法
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
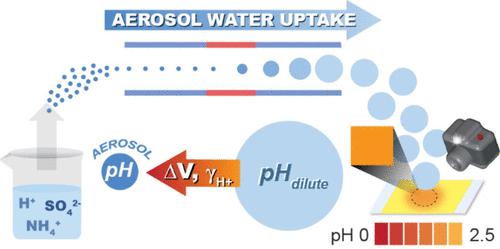
暴露于高浓度的大气气溶胶导致全球每年有数百万人死于呼吸系统和心血管疾病。由于大气气溶胶是强酸性的(−1 < pH < 5,平均≈2),酸催化的涉及半挥发性有机物质的多相反应是形成额外颗粒相质量的关键途径。然而,由于颗粒尺寸小(大气模式~ 100 nm)、含水量低以及H+离子的非保守性,直接气溶胶pH测量极具挑战性。在pH值为2的气溶胶中尤其如此,其中离子强度为10mol / l是常见的。广泛用于预测气溶胶pH值的热力学模型在低pH值下具有有限的基于测量的验证和挣扎,需要新的实验方法。在此,我们提出了一种测量气溶胶pH值的方法,该方法可在较宽的pH范围内(- 2至4)测量气溶胶pH值,包括pH <; 0难以接近的区域。悬浮颗粒在精确控制的过饱和环境中生长到均匀直径(4.8 μm),然后用酸化硫酸铵(AAS)撞击比色pH指示剂。这种稀释为指示剂引入了足够的液态水,并将pH值增加到比色可测量的范围。我们的研究结果表明,气溶胶的酸性可以比原始溶液高3个pH单位,与较低pH下的溶液的偏差越来越大。测量的气溶胶也比热力学模型预测的更酸。该方法的初步结果将使pH值测量在复杂的大气室实验和现实世界的位置,pH值仍然高度不确定和约束差,解决准确的气溶胶酸度值预测二次气溶胶形成的关键需求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Expanding Aerosol Acidity Measurements to the Water-Limited Regime: A Coupled Condensational Growth and Colorimetric Approach
Exposure to elevated concentrations of atmospheric aerosols leads to millions of deaths globally per year from respiratory and cardiovascular diseases. Acid-catalyzed multiphase reactions involving semivolatile organic species are a key pathway for forming additional particle-phase mass, as atmospheric aerosols are highly acidic (−1 < pH < 5, average ≈ 2). However, direct aerosol pH measurements are extremely challenging due to small particle sizes (atmospheric mode ∼100 nm), low water content, and the nonconservative nature of the H+ ion. This is particularly true in aerosols with pH < 2 where ionic strengths >10 molal are common. Widely used thermodynamic models for predicting aerosol pH have limited measurement-based validation and struggle at low pH, necessitating new experimental approaches. Herein, we present a method for measuring aerosol pH across a wide pH range (−2 to 4), including the difficult-to-access regime where pH < 0. Suspended particles are grown in a precisely controlled, supersaturated environment to a uniform diameter (4.8 μm) prior to impaction on a colorimetric pH indicator, with acidified ammonium sulfate (AAS). This dilution introduces sufficient liquid water for the indicator and increases the pH into the colorimetrically-measurable range. Our results demonstrate that aerosols can be more acidic than the original solution by up to 3 pH units, with increasing deviations from solution at lower pH. Measured aerosols were also more acidic than thermodynamic model predictions. Initial results with this method will enable pH measurement in complex atmospheric chamber experiments and real-world locations where pH values remain highly uncertain and poorly constrained, addressing the critical need for accurate aerosol acidity values to predict secondary aerosol formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


