酶解辅助糖基化对分离乳清蛋白致敏性和消化特性的影响
IF 8
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
在本研究中,乳清分离蛋白(WPI)用碱性蛋白酶适度水解,然后用allose糖基化,得到酶水解辅助糖基化的WPI。用光谱学和色谱法对改性蛋白的结构变化进行了表征。质谱鉴定和比较体外消化肽,以探索修饰的WPI的致敏性变化和抗原表位。结果表明,酶解辅助糖基化可显著降低WPI中α-乳清蛋白(α-LA)和β-乳球蛋白(β-LG)的致敏性。经胃肠道消化后,α-LA和β-LG的抗原性分别下降96.32±1.41%和94.95±1.92%。这是由于酶解预处理诱导了WPI的结构变化,从而增加了糖基化位点,降低了其致敏性。消化产物的肽鉴定显示,与仅糖基化(All-WPI)组相比,酶预处理的糖基化(E-All-WPI)组减少了37个致敏肽。E-All-WPI α-LA表位文库中未检测到致敏肽。最后,对消化的All-WPI和E-All-WPI共有的肽进行分子对接,探索糖基化/非糖基化肽与MHC-II在免疫应答中的相互作用。我们发现α-LA中的AA114-121肽和β-LG中的AA59-72肽在与MHC-II糖基化后,结合能分别降低了0.4 kJ/mol和2.4 kJ/mol。因此,糖基化干扰抗原与免疫因子的结合,从而抑制致敏性表达。本研究为WPI提供了一种新的改性方法,为进一步降低WPI的致敏性及其在食品工业中的应用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
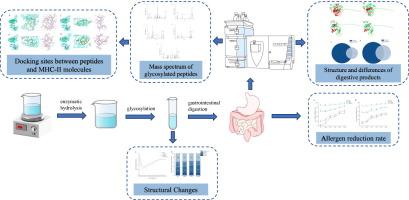
The effect of enzymatic hydrolysis assisted glycosylation on the allergenicity and digestive characteristics of whey protein isolate
In this study, whey protein isolate (WPI) was moderately hydrolyzed with alkaline protease, then glycosylated with allose to yield enzyme hydrolysis-assisted glycosylated WPI. The structural changes of the modified protein were characterized by spectroscopy and chromatography. Mass spectrometry identified and compared in vitro digestion peptides to explore modified WPI's allergenicity changes and antigenic epitopes. The results indicated that the enzyme hydrolysis-assisted glycosylation could significantly reduce the allergenicity of α-lactalbumin (α-LA) and β-lactoglobulin (β-LG) in WPI. After gastrointestinal digestion, the antigenicity of α-LA and β-LG decreased up to 96.32 ± 1.41 % and 94.95 ± 1.92 %. This is attributed to the fact that the pretreatment with enzymatic hydrolysis induces structural changes in WPI, thereby increasing the glycosylation sites and reducing its allergenicity. Peptide identification in digestion products showed 37 fewer allergenic peptides in enzyme-pretreated glycosylated (E-All-WPI) compared with glycosylation-only (All-WPI) groups. Moreover, no allergenic peptides were detected in E-All-WPI's α-LA epitope library. Finally, molecular docking of peptides common to digested All-WPI and E-All-WPI explored interactions between glycosylated/non-glycosylated peptides and MHC-II in immune responses. We found that the binding energies of AA114–121 peptides in α-LA and AA59–72 peptides in β-LG were reduced by 0.4 kJ/mol and 2.4 kJ/mol, respectively, after glycosylation with MHC-II. Thus, glycosylation interferes with the binding of antigens to immune factors, thereby inhibiting allergenic expression. This study provides a novel modification method for WPI, as well as new insights into further reducing WPI allergenicity and its applications in the food industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


