冷冻诱导卵黄颗粒蛋白质组的变化:无标记蛋白质组学和分子动力学模拟分析
IF 8
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
冷冻诱导的蛋黄颗粒变化显著影响了冻融蛋黄的功能特性。为了阐明这一现象背后的分子机制,本研究采用蛋白质组学分析和分子动力学(MD)模拟相结合的综合方法研究了冷冻诱导的蛋黄颗粒中蛋白质组的变化。结果发现低密度脂蛋白(LDL)、高密度脂蛋白(HDL)、卵黄免疫球蛋白(IgY)、核黄素结合蛋白(RBP)和磷维素(PV)是导致冷冻相关结构改变的关键蛋白。在27°C和- 20°C下进行的MD模拟表明,溶解度降低主要是由于疏水相互作用和氢键。介导这些相互作用的关键残基包括LDL中的VAL/ILE/LEU/GLN/ALA/SER簇(如VAL-89、ILE-82、LEU-60), HDL中的LEU-200/THR-197, IgY中的VAL-374/433, RBP中的THR/SER/LYS/ASN残基(如THR-56、ASN-18), PV中的GLU/PRO/LYS/ILE基序(如GLU-26、ILE-7)。这些发现表明,冻融诱导的蛋黄凝胶是由多个蛋白质组分之间的协同相互作用控制的,而不是孤立的分子事件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
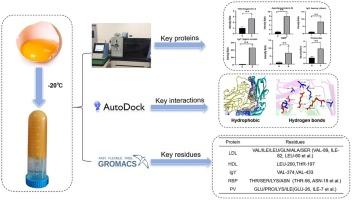
Changes in the proteome of yolk granules induced by freezing: Label-free proteomics and molecular dynamics simulation analysis
Freezing-induced changes of the egg yolk granules significantly compromises the functional properties of freeze-thawed egg yolk. To elucidate the molecular mechanisms underlying this phenomenon, this study employed an integrative approach combining proteomic analysis and molecular dynamics (MD) simulations to investigate freezing-induced proteome changes in egg yolk granules. The results identify low-density lipoprotein (LDL), high-density lipoprotein (HDL), yolk immunoglobulin (IgY), riboflavin-binding protein (RBP), and phosvitin (PV) as key proteins contributors to freezing-related structural alterations. MD simulations conducted at 27 °C and −20 °C revealed that reduced solubility primarily arises from hydrophobic interactions and hydrogen bonding. Critical residues mediating these interactions include VAL/ILE/LEU/GLN/ALA/SER clusters in LDL (e.g., VAL-89, ILE-82, LEU-60), LEU-200/THR-197 in HDL, VAL-374/433 in IgY, THR/SER/LYS/ASN residues (e.g., THR-56, ASN-18) in RBP, and GLU/PRO/LYS/ILE motifs (e.g., GLU-26, ILE-7) in PV. These findings demonstrate that freeze-thaw-induced gelation in egg yolk is governed by synergistic interactions across multiple protein components rather than isolated molecular events.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


