局部谷氨酰胺泄漏驱动根系微生物定植的空间结构
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
植物根系释放分泌物促进微生物群聚集,影响植物的功能和抗逆性。具体的渗出物是如何驱动空间殖民模式的,这在很大程度上仍是未知的。在本研究中,我们证明了形成根细胞外扩散屏障的内胚层Casparian条限制营养物质向根际的泄漏,与根际细菌的空间定植模式一致并控制着它们。我们发现血管源性谷氨酰胺渗漏是一种主要的细菌化学引诱剂和增殖促进剂,定义了一种以前未知的根分泌物形成途径。氨基酸化学感知缺陷的细菌对渗漏部位的吸引力降低,具有卡斯帕里斯条带缺陷的根显示细菌过度增殖,这取决于细菌对氨基酸代谢的能力。相关的慢性免疫刺激表明,内胚层营养限制对于调节微生物定植和组装至关重要,限制可能损害植物健康的过度增殖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
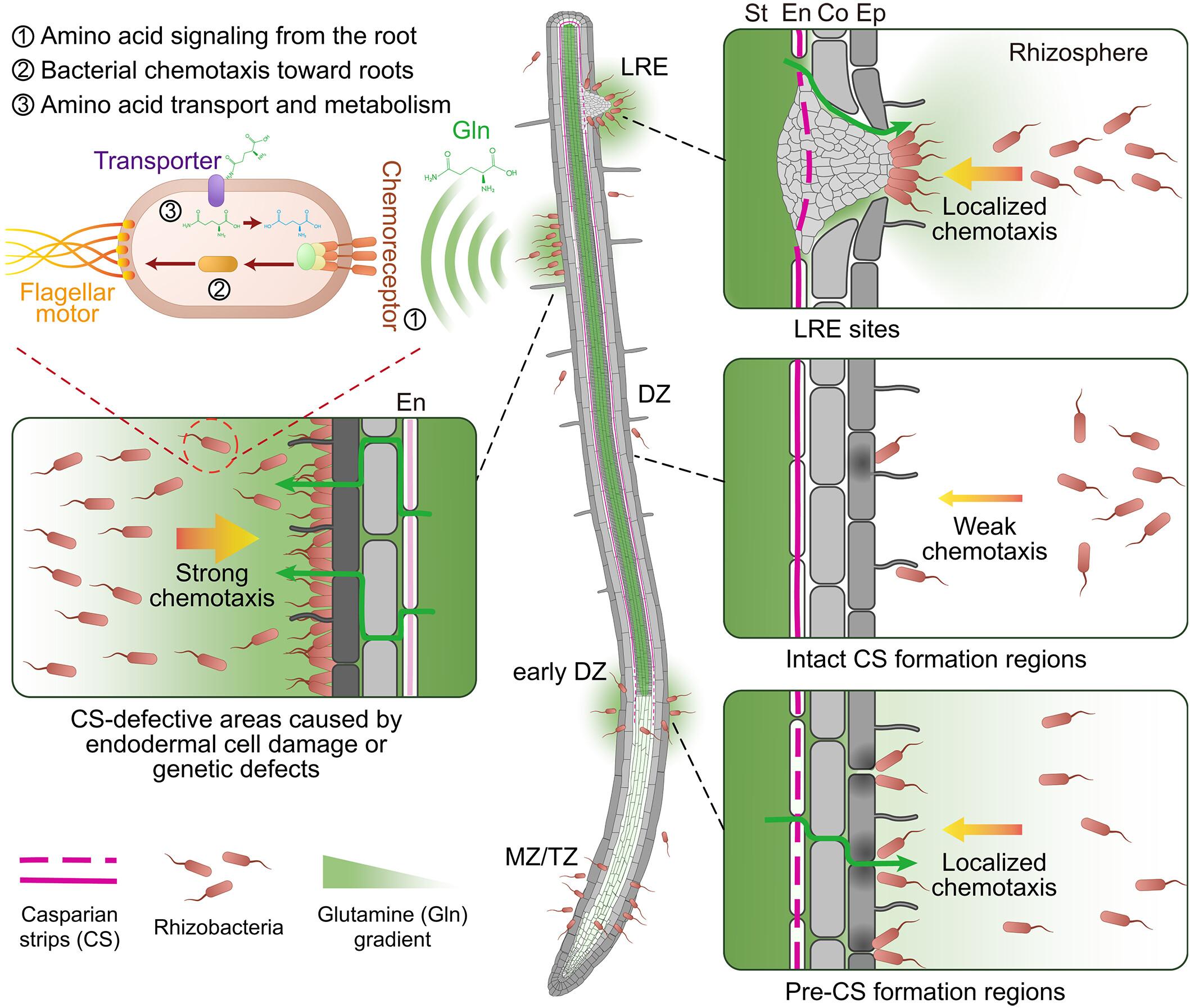
Localized glutamine leakage drives the spatial structure of root microbial colonization
Plant roots release exudates to encourage microbiome assembly, which influences the function and stress resilience of plants. How specific exudates drive spatial colonization patterns remains largely unknown. In this study, we demonstrate that endodermal Casparian strips—forming the root’s extracellular diffusion barrier—restrict nutrient leakage into the rhizosphere, coinciding with and controlling spatial colonization patterns of rhizobacteria. We find that vasculature-derived glutamine leakage is a major bacterial chemoattractant and enhancer of proliferation, defining a previously unknown pathway for root exudate formation. Bacteria defective in amino acid chemoperception display reduced attraction toward leakage sites, and roots with Casparian strip defects display bacterial overproliferation, dependent on bacterial capacity for amino acid metabolization. Associated chronic immune stimulation suggests that endodermal nutrient restriction is crucial for regulating microbial colonization and assembly, limiting excessive proliferation that could compromise plant health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


