通过树突纳米管网络的大脑细胞间通讯
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
细胞间纳米管网络介导物质交换,但它们在神经元中的存在还有待进一步研究。我们在哺乳动物皮层中发现了长而薄的树突丝状伪足形成直接树突-树突纳米管(DNTs)。解离神经元的超分辨率显微镜显示,DNTs具有富含肌动蛋白的成分和动力学特性,能够实现钙离子(ca2 +)的远程传播。成像和基于机器学习的分析验证了原位DNTs在解剖学上与突触棘不同。DNTs积极转运小分子和人淀粉样蛋白-β (Aβ);APP/PS1小鼠内侧前额叶皮层(APP, a β前体蛋白;PS1,早老素-1)的DNT密度在斑块形成前增加,提示树突-DNT网络可能在阿尔茨海默病病理中发挥作用。dnt介导的Aβ增殖的计算模型再现了早期淀粉样变性,预测了选择性的细胞内积累。这些发现揭示了大脑中的纳米管连接层,将神经元通信扩展到经典突触之外。本文章由计算机程序翻译,如有差异,请以英文原文为准。
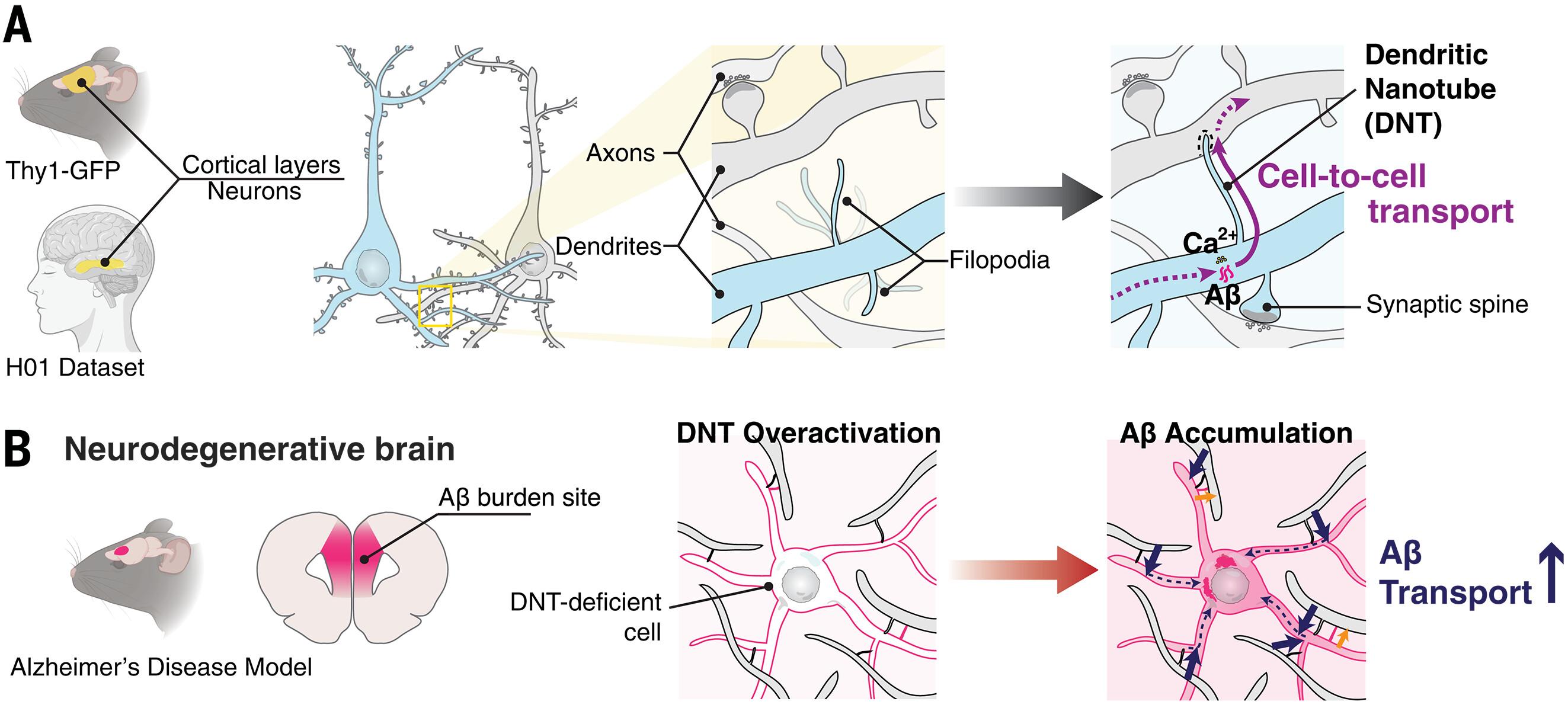
Intercellular communication in the brain through a dendritic nanotubular network
Intercellular nanotubular networks mediate material exchange, but their existence in neurons remains to be explored in detail. We identified long, thin dendritic filopodia forming direct dendrite–dendrite nanotubes (DNTs) in mammalian cortex. Super-resolution microscopy in dissociated neurons revealed DNTs’ actin-rich composition and dynamics, enabling long-range calcium ion (Ca2+) propagation. Imaging and machine learning–based analysis validated in situ DNTs as anatomically distinct from synaptic spines. DNTs actively transported small molecules and human amyloid-β (Aβ); DNT density increased before plaque formation in the medial prefrontal cortex of APP/PS1 mice (APP, Aβ precursor protein; PS1, presenilin-1), suggesting that the dendrite-DNT network might play a role in Alzheimer’s disease pathology. Computational models of DNT-mediated Aβ propagation recapitulated early amyloidosis, predicting selective intracellular accumulation. These findings uncover a nanotubular connectivity layer in the brain, extending neuronal communication beyond classical synapses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


