揭示肉桂醛与消化蛋白酶的结合景观:对特定相互作用和构象动力学的探索
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
肉桂醛(CMA)是一种具有健康益处的天然化合物,在食品和制药中已显示出多种生物效应。本文采用多光谱结合计算模拟的方法,系统研究了CMA与胰蛋白酶和胃蛋白酶的体外结合机制。结果表明,CMA与胰蛋白酶和胃蛋白酶的猝灭机制为静态猝灭机制。热力学参数、分子对接和动力学模拟分析表明,氢键和范德华力是cma -胰蛋白酶体系的主要相互作用力,而cma -胃蛋白酶体系的结合以静电力为主。在310 K时,cma -胃蛋白酶的结合亲和力显著高于cma -胰蛋白酶。光谱学分析证实了CMA能够诱导两者的构象变化。这些研究揭示了CMA与胰蛋白酶/胃蛋白酶的结合机制,为阐明CMA在消化系统中的潜在生物学作用提供了分子水平上的理论支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
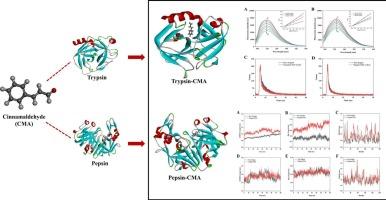
Unveiling the binding landscape of cinnamaldehyde with digestive proteases:An exploration of specific interactions and conformational dynamics
Cinnamaldehyde (CMA) is a natural compound with health benefits that have demonstrated multiple biological effects in food and pharmacy. Here, the binding mechanism of CMA to trypsin and pepsin in vitro was systematically investigated by multispectral combined with computational simulations. Results showed that CMA with trypsin and pepsin was static quenching mechanism. Thermodynamic parameters, molecular docking and dynamics simulation analyses showed that hydrogen bonding and van der Waals force were the main interaction forces in the CMA-trypsin system, whereas the binding of the CMA-pepsin system was dominated by electrostatic force. The binding affinity of the CMA-pepsin was significantly higher than that of the CMA-trypsin at 310 K. Spectroscopy analyses confirmed the ability of CMA to induce conformational changes in both. The studies have revealed the binding mechanism of CMA to trypsin/pepsin and provided theoretical support at the molecular level for elucidating the potential biological effects of CMA in the digestive system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


