5,7-二甲基化花青素的合成与性质:以芍药苷-3- o -葡萄糖苷和牵牛花苷-3- o -葡萄糖苷为重点
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
为了改善花青素脂溶性差和不稳定性的问题,本研究开发了一种高效的5,7-二甲基化花青素(Pn3GdM和Pt3GdM)的化学合成方法。采用全合成法对A环进行选择性甲基化,并由硫酸二甲酯介导甲氧基保护。通过高效液相色谱、核磁共振和质谱的结构和纯度表征证实了该策略的可行性,产品纯度超过95% %。甲基化花青素的疏水性显著增强,其logP值显著增加:Pn3G从- 0.118增加到- 0.026,Pt3G从- 0.806增加到- 0.194,同时保持了水溶性。此外,Pn3G的光稳定性也得到了显著改善。对比分析表明,b环取代基对抗氧化活性、颜色和pH稳定性的影响更大。这些结果表明,a环甲基化是一种很有前途的策略,可以提高花青素的溶解度和稳定性,在食品工业中有更广泛的应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
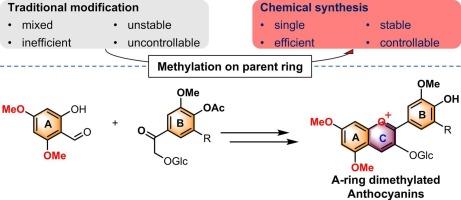
Synthesis and properties of 5,7-dimethylated anthocyanins: Focusing on peonidin-3-O-glucoside and petunidin-3-O-glucoside
This study developed an efficient chemical synthesis method for 5,7-dimethylated anthocyanins (Pn3GdM and Pt3GdM) to improve the poor liposolubility and instability of anthocyanins. The synthesis involved selective methylation of the A ring using a total synthesis approach and methoxy protection mediated by dimethyl sulfate. Structural and purity characterization by HPLC, NMR, and HRMS confirmed the feasibility of the strategy, yielding products with purities exceeding 95 %. The hydrophobicity of the methylated anthocyanins was significantly enhanced, as evidenced by increased logP values: Pn3G from −0.118 to −0.026 and Pt3G from −0.806 to −0.194, while maintaining water solubility. Additionally, the photostability of Pn3G was notably improved. Comparative analyses revealed that B-ring substituents had a greater impact on antioxidant activity, color, and pH stability. These results demonstrate that A-ring methylation is a promising strategy to enhance the solubility and stability of anthocyanins, offering potential for broader applications in food industries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


