κ-卡拉胶酶MtKC16A在枯草芽孢杆菌中分泌性表达制备κ-卡拉胶
IF 5.9
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
卡拉胶酶可以降解卡拉胶并产生偶数新卡拉胶寡糖(ncos)。然而,κ-卡拉胶的凝胶特性限制了κ-卡拉胶酶能处理的最大底物浓度。本研究首先在枯草芽孢杆菌中构建了表达体系,实现了分泌产生nκ 4的κ-卡拉胶酶MtKC16A,活性为0.016 U/mL。我们进一步的研究表明,MtKC16A还可以从κ- carrara7 - taose、κ-carranonaose、κ- carraunaase和κ- carrauntetradecase的非还原端切割三糖单元。这使我们成功地通过酸水解和酶水解相结合制备了κ-卡拉糖。1 g κ-卡拉胶可得0.47±0.01 g κ-卡拉胶糖,收率高达47.0% (w/w)。此外,我们的研究表明,酸水解与酶水解相结合制备的卡拉胶寡糖(COSs)具有良好的体外抗氧化活性,显著高于多糖。本研究提出了利用κ-卡拉胶酶高效制备奇数cos的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
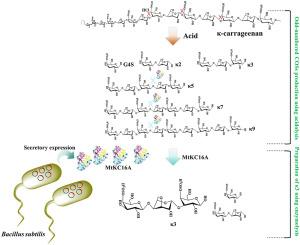
Secretory expression of κ-carrageenase MtKC16A in Bacillus subtilis for the preparation of κ-carratriose
Carrageenases can degrade carrageenan and produce even-numbered neocarrageenan-oligosaccharides (NCOSs). However, the gel properties of κ-carrageenan limit the maximum concentration of substrate that can be treated by κ-carrageenase. In this study, we first constructed an expression system in Bacillus subtilis and achieved secretion of Nκ4-producing κ-carrageenase MtKC16A, with an activity of 0.016 U/mL. Our further exploration showed that MtKC16A can also cleave the trisaccharide unit from the non-reducing end of κ-carraheptaose, κ-carranonaose, κ-carraundecaose, and κ-carrauntetradecaose. This allowed us to successfully prepare κ-carratriose by combining acid hydrolysis and enzymatic hydrolysis. We were able to obtain 0.47 ± 0.01 g of κ-carratriose from 1 g of κ-carrageenan, with a high yield of 47.0 % (w/w). Furthermore, our study demonstrated that carrageenanoligosaccharides (COSs) prepared by acid hydrolysis combined with enzymatic hydrolysis have excellent in vitro antioxidant activity, significantly higher than polysaccharides. This study presents new methods for the efficient preparation of odd-numbered COSs using κ-carrageenases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Bioscience
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
6.40
自引率
5.80%
发文量
671
审稿时长
27 days
期刊介绍:
Food Bioscience is a peer-reviewed journal that aims to provide a forum for recent developments in the field of bio-related food research. The journal focuses on both fundamental and applied research worldwide, with special attention to ethnic and cultural aspects of food bioresearch.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


