双重增强猪肉颜色稳定性:l -组氨酸/ l -精氨酸介导的抗氧化活性和肌红蛋白结构致密性
IF 8.2
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
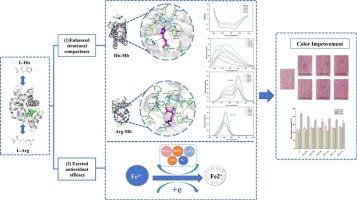
本研究旨在探讨l -组氨酸(His)和l -精氨酸(Arg)对猪肉色泽的影响,并揭示其可能的作用机制。0.010 ~ 0.30% His处理的a*值分别提高2.36%、4.78%和8.65%,0.10 ~ 0.30% Arg处理的a*值分别提高3.45%、8.40%和13.06% (P < 0.05)。他/精氨酸增加氧合肌红蛋白水平,同时降低肌红蛋白水平呈剂量依赖性。同时,His/Arg增加了巯基含量,降低了活性氧、TBARS和羰基水平(P < 0.05)。多光谱分析表明,His/Arg主要通过与酪氨酸残基的相互作用,增加了α-螺旋的含量,抑制了肌红蛋白三级结构的展开。分子对接模拟表明,His/Arg可以通过氢键和疏水相互作用与肌红蛋白形成稳定的复合物。这些变化可能产生一个保护屏障来限制肌红蛋白氧化,从而增强颜色稳定性。总体而言,His/Arg通过发挥抗氧化活性和增强肌红蛋白的结构致密性来增强猪肉的颜色稳定性,其中Arg的效果优于His。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual enhancement of pork color stability: Antioxidant activity and myoglobin structural compactness mediated by L-histidine/L-arginine
This study aimed to investigate the effect of L-histidine (His) and L-arginine (Arg) on the color of pork and reveal the possible mechanism. The a* value increased by 2.36 %, 4.78 % and 8.65 % with 0.10–0.30 % His, and by 3.45 %, 8.40 % and 13.06 % with 0.10–0.30 % Arg, respectively (P < 0.05). His/Arg increased oxymyoglobin levels while reducing metmyoglobin levels in dose-dependent patterns. Concomitantly, His/Arg increased sulfhydryl content and decreased reactive oxygen species, TBARS, and carbonyl levels (P < 0.05). Multi-spectral analyses revealed that His/Arg increased the α-helix content and inhibited the unfolding of tertiary structure of myoglobin mainly by interactions with tyrosine residues. Molecular docking simulations suggested that His/Arg could form stable complexes with myoglobin through hydrogen bonding and hydrophobic interactions. These changes might create a protective barrier to limit myoglobin oxidation, thus enhancing color stability. Overall, His/Arg enhanced pork color stability by exerting antioxidant activity and strengthening structural compactness of myoglobin, with Arg demonstrating greater efficacy than His.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry: X
CHEMISTRY, APPLIED-
CiteScore
4.90
自引率
6.60%
发文量
315
审稿时长
55 days
期刊介绍:
Food Chemistry: X, one of three Open Access companion journals to Food Chemistry, follows the same aims, scope, and peer-review process. It focuses on papers advancing food and biochemistry or analytical methods, prioritizing research novelty. Manuscript evaluation considers novelty, scientific rigor, field advancement, and reader interest. Excluded are studies on food molecular sciences or disease cure/prevention. Topics include food component chemistry, bioactives, processing effects, additives, contaminants, and analytical methods. The journal welcome Analytical Papers addressing food microbiology, sensory aspects, and more, emphasizing new methods with robust validation and applicability to diverse foods or regions.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


