含吡唑酰胺结构黄酮醇衍生物的设计、合成及生物活性研究
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
合成了一系列吡唑酰胺类黄酮醇衍生物,并对9种真菌进行了抑菌活性测试。多数化合物对辣椒疫霉有较强的抑制作用。值得注意的是,J13对Pc的抑制作用最高(EC50 = 6.29 μg/mL),显著优于azoxystrobin (96.5 μg/mL)和fluopyram (FI, 127.95 μg/mL)。用J13对辣椒果实、叶片和茄子进行了体内抑菌试验。辣椒叶片的体内实验表明,J13 (200 μg/mL)的治疗作用(89.4%)和保护作用(87.2%)优于FI(47.9%和33.7%)。扫描电镜(SEM)和荧光显微镜(FM)观察到J13对Pc菌丝细胞膜的破坏作用。通过检测细胞质渗漏、评估膜通透性和测量丙二醛(MDA)含量,进一步证实了这种膜破坏。此外,J13诱导线粒体功能障碍,表现为线粒体膜电位改变和琥珀酸脱氢酶(SDH)活性降低;分子对接和分子动力学模拟显示J13与SDH蛋白的稳定结合。此外,J13对靶植物和非靶生物均表现出良好的生物安全性。这些实验结果为开发新型环境友好型杀菌剂的创新策略奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
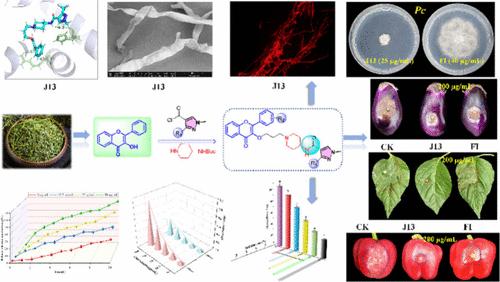
Design, Synthesis, and Biological Activity Studies of Flavonol Derivatives Containing Pyrazolamide Structure
A series of flavonol derivatives with pyrazole amide were synthesized, and their antifungal activity was tested against 9 fungi in this study. Most compounds showed strong inhibitory effect against Phytophthora capsici. Notably, J13 exhibited the highest inhibition against Pc (EC50 = 6.29 μg/mL), significantly outperforming azoxystrobin (96.5 μg/mL) and fluopyram (FI, 127.95 μg/mL). In vivo antioomycete experiments with J13 were conducted on chili fruits, leaves, and eggplant. In vivo tests on chili leaves revealed that J13 (200 μg/mL) provided superior curative (89.4%) and protective (87.2%) effects compared to FI (47.9 and 33.7%). J13 caused damage to the cell membranes of Pc hyphae, as observed in scanning electron microscopy (SEM) and fluorescence microscopy (FM) experiments. This membrane disruption was further confirmed by detecting cytoplasmic leakage, assessing membrane permeability, and measuring the malondialdehyde (MDA) content. Furthermore, J13 induced mitochondrial dysfunction, manifested as altered mitochondrial membrane potential and reduced succinate dehydrogenase (SDH) activity; molecular docking and molecular dynamics simulations demonstrated stable binding of J13 to the SDH protein. Moreover, J13 demonstrated favorable biosafety toward both target plants and nontarget organisms. These experimental results lay the groundwork for innovative strategies to develop novel environmentally friendly fungicides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


