左氧氟沙星诱导肝损伤的综合分析:来自药物警戒、人肝类器官和细胞外囊泡蛋白质组学的见解
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
背景:左氧氟沙星(LVX)是一种广泛使用的氟喹诺酮类抗生素,与类似物曲瓦沙星(TVX)相比,通常被认为具有较低的肝毒性。虽然LVX被认为是相对安全的,但它与药物性肝损伤(DILI)病例有关,因此需要对其进行全面的机制评估。方法本研究采用基于FDA不良事件报告系统(FAERS)、人肝类器官建模和细胞外囊泡(EV)蛋白质组学的真实世界药物警戒分析相结合的综合策略,系统评估lvx相关的DILI风险和机制。结果FAERS共发现1671例lvx相关DILI病例,以男性和45-65岁人群为主。歧化分析显示有统计学意义的胆汁淤积性肝损伤表型信号,包括混合性肝损伤、胆汁淤积、结合性高胆红素血症等。肝类器官实验显示,LVX诱导的肝细胞损伤程度较TVX轻。来自lvx暴露的类器官的EV蛋白质组学分析发现糖酵解是最显著富集的途径,磷酸甘油酸激酶1 (PGK1)、乳酸脱氢酶A (LDHA)和果糖二磷酸醛缩酶A (ALDOA)显著上调。通过RT-qPCR和蛋白质组学分析进一步验证了这三个糖酵解相关关键靶点的表达,分子对接表明LVX与这些蛋白具有较强的结合亲和力。综上所述,这些发现表明糖酵解重编程可能参与了lvx诱导的肝损伤的发病机制。本研究通过将现实世界的药物警戒数据与人类肝脏类器官模型相结合,提出了一种多维度的策略来研究lvx诱导的肝损伤。总之,这些发现为阐明DILI提供了一个翻译框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
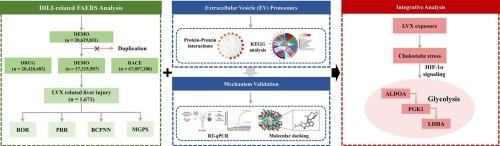
Integrative analysis of levofloxacin-induced liver injury: Insights from pharmacovigilance, human liver organoids, and extracellular vesicle proteomics
Background
Levofloxacin (LVX), a widely used fluoroquinolone antibiotic, is generally considered to have low hepatotoxic potential compared to its analog trovafloxacin (TVX). Although considered relatively safe, LVX has been implicated in Drug-induced liver injury (DILI) cases, prompting the need for a comprehensive mechanistic assessment.
Methods
This study employed an integrated strategy combining real-world pharmacovigilance analysis based on FDA Adverse Event Reporting System (FAERS), human liver organoid modeling, and extracellular vesicle (EV) proteomics to systematically assess LVX-associated DILI risks and mechanisms.
Results
A total of 1671 LVX-related DILI cases were identified in FAERS, with a predominance in males and individuals aged 45–65. Disproportionality analysis revealed statistically significant signals indicative of cholestatic liver injury phenotypes, including mixed liver injury, cholestasis, and conjugated hyperbilirubinemia, etc. Furthermore, liver organoid assays revealed that LVX induced moderate hepatocellular injury, which was less severe than that caused by TVX. EV proteomic analysis from LVX-exposed organoids identified glycolysis as the most significantly enriched pathway, with notable upregulation of phosphoglycerate kinase 1 (PGK1), lactate dehydrogenase A (LDHA), and fructose-bisphosphate aldolase A (ALDOA). The expression of these three key glycolysis-related targets was further validated by RT-qPCR and proteomic analysis, and molecular docking demonstrated strong binding affinities between LVX and these proteins. Collectively, these findings suggest that glycolytic reprogramming may contribute to the pathogenesis of LVX-induced liver injury.
Conclusions
This study presents a multidimensional strategy to investigate LVX-induced liver injury by integrating real-world pharmacovigilance data with a human liver organoid model. Together, these findings provide a translational framework for elucidating DILI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


