羟基和三氟甲基自由基碳水化合物足迹探测低聚糖蛋白质结合成分
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
碳水化合物以各种形式存在于生物体中,既可以是独立的聚糖,也可以是糖缀合物,包括糖蛋白、糖脂和糖胺聚糖。这些结构在许多生物过程中起着至关重要的作用,通常由碳水化合物与其他生物分子的相互作用介导或影响。然而,由于碳水化合物的结构复杂性、动态构象行为以及通常涉及的低结合亲和,研究这些相互作用尤其具有挑战性。为了应对这些挑战,我们正在开发一种利用基于质谱的自由基碳水化合物足迹(RCF)的新方法。我们通过测量羟基自由基和三氟甲基自由基介导氧化的表观速率的变化来监测低聚糖内特定区域的溶剂可及性的变化。在我们的研究中,一组三糖异构体和N,N ',N″-三乙酰壳三糖(NAG3)在非结合蛋白溶液中的修饰没有明显变化。然而,在两种特异性结合NAG3的蛋白的存在下,NAG3的氧化被减少。我们发现,在共价标记条件下,自由还原端是羟基自由基氧化的主要位点,使其能够区分聚糖还原端的相互作用。相反,三氟甲基自由基通过取代成碳氢键,在三糖上广泛地标记。总的来说,这种方法为鉴定聚糖-蛋白质相互作用和绘制聚糖结合界面提供了一种强大的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
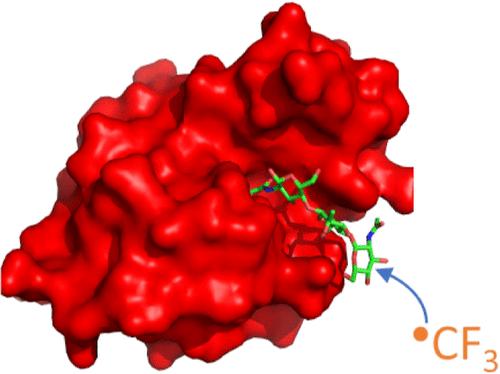
Hydroxyl and Trifluoromethyl Radical Carbohydrate Footprinting for Probing Protein Binding Components of Oligosaccharides
Carbohydrates are found in various forms in living organisms, both as free-standing glycans as well as glycoconjugates including glycoproteins, glycolipids, and glycosaminoglycans. These structures play crucial roles in many biological processes, often mediated or influenced by interactions of carbohydrates with other biomolecules. However, studying these interactions is particularly challenging due to the structural complexity of carbohydrates, their dynamic conformational behavior, and the low binding affinities often involved. To address these challenges, we are developing a novel method that leverages mass spectrometry-based radical carbohydrate footprinting (RCF). We monitored changes in the solvent accessibility of specific regions within oligosaccharides by measuring variations in the apparent rate of hydroxyl radical and trifluoromethyl radical-mediated oxidation. In our studies, a collection of trisaccharide isomers and N,N′,N″-triacetylchitotriose (NAG3) shows no significant change in modification in nonbinding protein solutions. However, in the presence of two proteins that bind NAG3 specifically, NAG3 oxidation is reduced. We find that the free reducing end is the primary site of hydroxyl radical oxidation under covalent labeling conditions, allowing it to distinguish interactions at the glycan reducing end. Trifluoromethyl radicals, conversely, label broadly across the trisaccharide by substitution into a C–H bond. Overall, this approach offers a powerful novel approach for identifying glycan-protein interactions and mapping the binding interface of glycans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


