二苯醚对成年斑马鱼和人淋巴细胞的毒理学评价:生化、基因毒性、细胞毒性、组织病理学和超微结构分析。
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本研究通过生化、基因毒性、组织病理学和超微结构分析,评估了二苯醚(DE)对成年斑马鱼鳃和血液的毒理学影响。此外,在人淋巴细胞中,使用染色体畸变和细胞分裂阻断微核(CBMN)测定等体外测定方法评估了DE的毒性作用。斑马鱼和人淋巴细胞的DE暴露浓度分别为2.13 mg/L(¼LC50)和4.25 mg/L(½LC50)。据报道,DE暴露后斑马鱼鳃中SOD、GST、AChE活性和MDA水平等生化参数发生了显著变化。结果表明,DE暴露后斑马鱼鳃细胞和血细胞的橄榄尾矩(OTM)显著升高。MN检测显示,DE暴露的斑马鱼红细胞胞浆异常(ECA)、红细胞核异常(ENA)和微核(MN)频率显著升高。斑马鱼暴露于DE后的鳃组织出现了不同类型的组织病理学改变,并通过扫描电镜分析进一步证实了这一点。对斑马鱼的红细胞进行扫描电镜分析,发现斑马鱼红细胞形态发生改变,红细胞形状不规则,边界不规则,红细胞肿胀等。此外,染色体畸变等体外实验显示,DE暴露于人淋巴细胞后,人血细胞出现多倍体、卫星关联、端粒关联、染色体断裂等不同类型的改变,出现多核细胞、晚期凋亡细胞、微核细胞等不同类型的异常。本文章由计算机程序翻译,如有差异,请以英文原文为准。
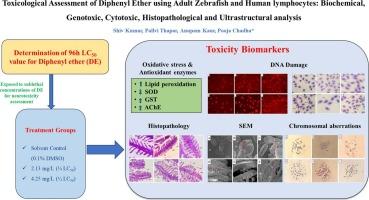
Toxicological assessment of diphenyl ether using adult zebrafish and human lymphocytes: Biochemical, genotoxic, cytotoxic, histopathological and ultrastructural analysis
In the present investigation, the toxicological impact of diphenyl ether (DE) was assessed in gills and blood of adult zebrafish through biochemical, genotoxic, histopathological and ultrastructural analysis. Moreover, in human lymphocytes, toxic effects of DE were evaluated using in vitro assays such as chromosomal aberration and cytokinesis-block micronucleus (CBMN) assay. The exposure concentrations of DE used for the toxicity assessment were 2.13 mg/L (¼ LC50) and 4.25 mg/L (½ LC50) for zebrafish as well as human lymphocytes. Significant changes in the biochemical parameters such as SOD, GST, AChE activity and MDA levels were reported after DE exposure in zebrafish gill. The olive tail moment (OTM) was found to be significantly increased in gill and blood cells of DE exposed zebrafish. The MN assay revealed significantly elevated frequency of erythrocytic cytoplasmic abnormalities (ECA), erythrocytic nuclear abnormalities (ENA) and micronuclei (MN) in the DE exposed zebrafish. Various types of histopathological alterations were reported in gill tissue of zebrafish exposed to DE which were further confirmed through SEM analysis. The SEM analysis of erythrocytes of zebrafish exposed to DE revealed the morphological alterations such as irregular shaped erythrocytes with irregular boundaries, swollen erythrocytes etc. Moreover, In vitro assay such as chromosomal aberration assay showed the different types alterations such as polyploidy, satellite association, telomeric association and chromosomal breakage in human blood cells exposed to DE. Different kinds of abnormalities such as multiple nucleated cells, late apoptotic cells, micronucleated cells etc. were reported after DE exposure to human lymphocytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


