在宫颈癌中,Caulerpin通过Hippo信号抑制肿瘤相关血管生成和肿瘤生长
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
Caulerpin是一种从藻属绿藻中提取的双吲哚类生物碱,在体外实验中对多种肿瘤细胞具有良好的抗增殖作用。然而,其药理潜力尚未深入探讨宫颈癌。本研究在宫颈癌细胞(HeLa和SiHa细胞)和异种移植小鼠模型中评估了caulerpin的抗肿瘤特性。研究发现,caulerpin显著抑制HeLa和SiHa细胞的生长、集落形成、迁移、侵袭和诱导细胞凋亡,抑制HeLa异种移植物体内肿瘤生长。在人脐静脉内皮细胞(HUVECs)中,caulerpin在(间接)或(直接)肿瘤条件培养基刺激下显著抑制增殖、迁移和管形成。在机械上,caulerpin通过抑制YAP1核易位和血管生成相关蛋白的表达,包括肿瘤细胞中HIF-1α和VEGFA的表达,HUVECs中VEGFR2和CD31的表达,阻断了体外和体内的血管生成。TDI-011536(一种Hippo特异性抑制剂)有效地逆转了caulerpin的作用。这些结果证明了caulerpin在宫颈癌中的抗血管生成作用,Hippo/YAP1通过VEGF/VEGFR2信号通路介导肿瘤-内皮细胞相互作用,支持其作为宫颈癌治疗的天然产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
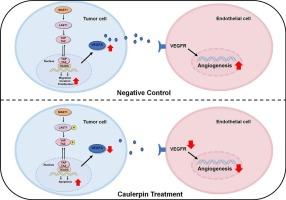
Caulerpin suppresses tumor-associated angiogenesis and tumor growth via Hippo signaling in cervical cancer
Caulerpin, a bisindole alkaloid derived from green algae of the genus Caulerpa, has exhibited a promising anti-proliferative effect on various tumor cells in vitro. However, its pharmacological potential has not been intensively explored in cervical cancer. In this study, the antitumor property of caulerpin was assessed in cervical cancer cells (HeLa and SiHa cells) and xenograft mouse models. It was found that caulerpin significantly inhibited cell growth, colony formation, migration, invasion and induced cell apoptosis in HeLa and SiHa cells, and inhibited the tumor growth of HeLa xenografts in vivo. In human umbilical vein endothelial cells (HUVECs), caulerpin significantly suppressed proliferation, migration, and tube formation with (indirect) or without (direct effects) tumor-conditioned medium stimulation. Mechanically, caulerpin treatment blocked angiogenesis in vitro and in vivo through suppressing YAP1 nuclear translocation and angiogenesis-related proteins expression, including HIF-1α and VEGFA expression in tumor cells, VEGFR2 and CD31 expression in HUVECs. TDI-011536 (a specific inhibitor of Hippo) effectively reversed the effect of caulerpin. These results demonstrate the anti-angiogenic effect of caulerpin in cervical cancer that Hippo/YAP1 mediates tumor-endothelial cell interaction via VEGF/VEGFR2 signaling, which supports it as a natural product for the management of cervical cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


