板蓝根中四种主要硫代葡萄糖苷的热稳定性及体内体外代谢研究
IF 8
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
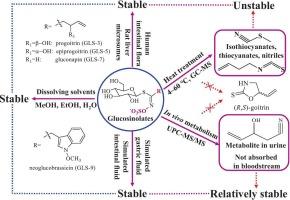
板蓝根中的硫代葡萄糖苷(gls)及其降解产物(如甲状腺素)因其抗病毒活性而受到广泛关注。gls在内源性黑芥子酶作用下的降解机制和产物已被明确。然而,目前尚不清楚蓝靛根部的gls是否可以进行热分解,或者在没有黑芥子酶的情况下,两种主要的gls(前甲状腺素和表甲状腺素)是否可以生物转化为生物活性的甲状腺素。本研究首次对四种主要gls(包括前高血糖素、表高血糖素、糖蛋白素和新糖花青素)的热稳定性和代谢行为进行了评价。葡糖苷在四种gls中表现出最强大的热稳定性,即使在100°C下也能保持其结构完整性。其余三种gls仅在较低温度(0-20°C)下稳定,在40°C时降解,在60°C时完全降解。三种gls的降解产物通过静态顶空气相色谱-质谱分析,鉴定为异硫氰酸酯、硫氰酸酯、腈和烯醇。这四种gls没有通过模拟胃液、模拟肠液、大鼠肝微粒体或人肠道菌群生物转化为各自的产物。在小鼠体内对脂肪族硫代葡萄糖苷类物质(前高血糖素、表高血糖素、葡萄糖糖苷和紫荆素)的代谢研究发现,UPLC-ESI-MS/MS在小鼠血浆中未检测到完整的GLS及其代谢物,表明GLS在血液中吸收不良。然而,在小鼠的尿液中仅鉴定出四种gls的一种常见的次要代谢物,以及大量完整的gls。利用已报道的gls的巯基酸代谢途径,LC-MS鉴定其代谢产物为n -乙酰半胱氨酸偶联物。本研究结果表明,gls主要以其原始形式在尿液中排泄,而不被吸收到血液中。综上所述,在缺乏黑芥子酶的情况下,在热应激或生理应激条件下,靛蓝根中的两种主要gls(前甲状腺素和表甲状腺素)不能转化为活性甲状腺素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermal stability, and in vitro and in vivo metabolism of four main glucosinolates in Isatis indigotica roots
The glucosinolates (GLSs) and their degradation products (such as goitrin) in Isatis indigotica roots have been the subject of considerable research interest due to their antiviral activities. The degradation mechanism and products of GLSs under the endogenous myrosinase are well-established. Nevertheless, it remains uncertain whether GLSs in I. indigotica roots can undergo thermal decomposition, or whether the two principal GLSs (progoitrin and epiprogoitrin) can biotransform into the bioactive goitrin in the absence of myrosinase. In this study, the thermal stability and metabolic behavior of four main GLSs (including progoitrin, epiprogoitrin, gluconapin, and neoglucobrassicin) were estimated for the first time. Gluconapin demonstrated the most robust thermal stability among the four GLSs, retaining its structural integrity even at 100 °C. The remaining three GLSs were found to be stable only at lower temperatures (0–20 °C), exhibiting degradation at 40 °C and complete degradation at 60 °C. The degraded products of the three GLSs were subjected to analysis by static headspace GC–MS, which were identified as isothiocyanates, thiocyanates, nitriles, and enols. The four GLSs were not biotransformed into their respective products by simulated gastric fluid, simulated intestinal fluid, rat liver microsomes, or human intestinal flora. The in vivo metabolism study of the aliphatic glucosinolates (progoitrin, epiprogoitrin, gluconapin, and sinigrin) in mice revealed that the intact GLS and their metabolites of these four GLSs were undetected in the mice plasma by UPLC-ESI-MS/MS, indicating that the GLS were poorly absorbed into the blood. However, only a common minor metabolite of the four GLSs were identified in the urine of the mice, along with abundant intact GLSs. The metabolite was identified as N-acetylcysteine conjugates by LC-MS, with the aid of the reported mercapturic acid metabolic pathway of GLSs. The findings of this study indicated that the GLSs are predominantly excreted in urine in their original forms and are not absorbed into the bloodstream. In conclusion, the two principal GLSs (progoitrin and epiprogoitrin) in I. indigotica roots are unable to undergo conversion to active goitrin under conditions of heat stress or physiological stress in the absence of myrosinase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Research International
工程技术-食品科技
CiteScore
12.50
自引率
7.40%
发文量
1183
审稿时长
79 days
期刊介绍:
Food Research International serves as a rapid dissemination platform for significant and impactful research in food science, technology, engineering, and nutrition. The journal focuses on publishing novel, high-quality, and high-impact review papers, original research papers, and letters to the editors across various disciplines in the science and technology of food. Additionally, it follows a policy of publishing special issues on topical and emergent subjects in food research or related areas. Selected, peer-reviewed papers from scientific meetings, workshops, and conferences on the science, technology, and engineering of foods are also featured in special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


