蛋白酶种类对大豆分离蛋白和绿豆蛋白有限酶解产物结构、界面行为和发泡性能的影响
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
植物蛋白泡沫虽然具有工业潜力,但在发泡能力(FC)方面存在局限性。本研究创新性地采用6种不同的蛋白酶(胰蛋白酶、Alcalase、木瓜蛋白酶、中和酶、复合蛋白酶、风味酶)对大豆分离蛋白(SPI)和绿豆蛋白(MBP)进行有限酶解,以提高其界面和发泡性能。关键是,水解显著降低了两种蛋白质的分子量和粒径,增加了分子柔韧性,降低了表面张力。这些结构的改变促进了空气-水界面的快速吸附和重排。因此,所有酶处理都显著提高了FC(胰蛋白酶使SPI FC最大化至167.33 %,Flavourzyme使MBP FC最大化至190.67 %),而不影响发泡稳定性。这项系统的研究在酶修饰、界面活性和增强的发泡性能之间建立了明确的结构功能关系。它为选择最佳蛋白酶来定制SPI和MBP功能提供了关键的见解,显着扩大了它们在泡沫食品中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
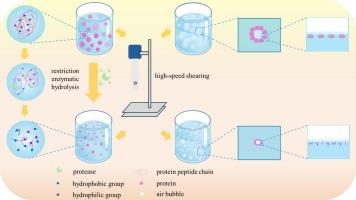
Effect of protease species on structure, interfacial behavior, and foaming properties of limited enzyme hydrolysis products of soybean protein isolate and mung bean protein
Plant protein foams face limitations in foaming capacity (FC) despite their industrial potential. This study innovatively employed six distinct proteases (Trypsin, Alcalase, Papain, Neutrase, Compound proteinase, Flavourzyme) for limited enzymatic hydrolysis of soy protein isolate (SPI) and mung bean protein (MBP) to enhance their interfacial and foaming properties. Crucially, hydrolysis significantly reduced molecular weight and particle size, increased molecular flexibility, and lowered surface tension for both proteins. These structural modifications facilitated rapid adsorption and rearrangement at the air-water interface. Consequently, all enzymatic treatments markedly enhanced FC (Trypsin maximized SPI FC to 167.33 %, Flavourzyme maximized MBP FC to 190.67 %) without compromising foaming stability. This systematic investigation establishes a clear structure-function relationship between enzymatic modification, interfacial activity, and enhanced foaming performance. It provides critical insights for selecting optimal proteases to tailor SPI and MBP functionality, significantly broadening their application in foam-based food products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


