从可再生木材中提取的生物炭在稳定的金属镁阳极上作为离子传导保护层
IF 9.7
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
可充电镁金属电池有望成为锂电池的潜在竞争对手。然而,Mg金属阳极很容易与传统的有机电解质钝化,导致离子阻断间相层的形成。在此,我们提出竹炭(BCs)作为Mg2+导电保护层在Mg金属表面。在这个过程中,已经证明,与苹果木炭(ACs)相比,BCs具有丰富的官能团,高度无序和大层间距的特点,具有出色的Mg2+导电性。此外,我们提出了一种易于加工的制备bc /Mg复合材料的方法,重点是避免在界面处产生副产物。由此形成的三维(3D) bc保护层系统有效地增加了离子传输通道的数量,从而提高了离子传输效率,同时减轻了电解质的分解。BCs/Mg阳极的Mg-Mg对称电池比使用普通电解质Mg(TFSI)2在乙腈中的裸Mg和ACs/Mg电极具有更低的过电位(~ 0.27 V)和界面阻抗。与其他两种电极相比,BCs/Mg||V2O5全电池在抗氧化电解质及其含水电解质中的循环稳定性、库伦效率(CE)和电压迟滞性都有很大提高。我们期望这项研究将通过开发适合需要界面保护的碱金属电池系统的无钝化阳极材料提供实验证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
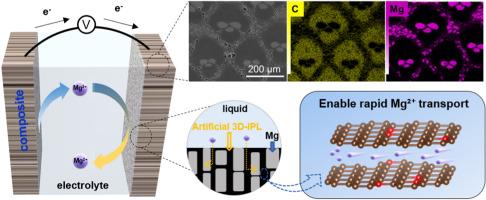
Biochar from renewable wood as an ion-conducting protective layer on stable magnesium metal anodes
Rechargeable Mg-metal batteries (RMBs) are expected to be a potential competitor for lithium (Li) counterparts. However, the Mg metal anode readily passivates with conventional organic electrolytes, leading to the formation of an ion-blocking interphase layer. Here, we propose that bamboo charcoals (BCs) used as a Mg2+-conducting protective layer on the surface of Mg metal. In this process, it has been demonstrated that compared to apple-wood charcoals (ACs), BCs, distinguished by their plentiful functional groups, high degree of disorder, and large layer spacing, exhibit outstanding Mg2+ conductivity. Furthermore, we have proposed a facile and processable method to fabricate BCs/Mg composite, with a focus on circumventing the generation of by-products at the interface. The resultant system of three-dimensional (3D) BCs protective layer effectively increases the number of ion transport channels, thereby boosting ion transport efficiency while concurrently mitigating electrolyte decomposition. The Mg-Mg symmetrical cell with BCs/Mg anode demonstrates lower overpotential (∼0.27 V) and interfacial impedance than bare Mg and ACs/Mg electrode using a common electrolyte of Mg(TFSI)2 in acetonitrile. The cycling stability, Coulombic efficiency (CE), and voltage hysteresis of the BCs/Mg||V2O5 full cell are greatly improved compared to the other two electrodes in both oxidation-resistant electrolyte and its water-containing electrolyte. We expect that this study will provide experimental substantiation by developing passivation-free anode materials tailored for alkali metal battery systems that require interface protection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Physics
Materials Science-General Materials Science
CiteScore
14.00
自引率
7.80%
发文量
284
审稿时长
15 days
期刊介绍:
Materials Today Physics is a multi-disciplinary journal focused on the physics of materials, encompassing both the physical properties and materials synthesis. Operating at the interface of physics and materials science, this journal covers one of the largest and most dynamic fields within physical science. The forefront research in materials physics is driving advancements in new materials, uncovering new physics, and fostering novel applications at an unprecedented pace.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


