基于蛋白质组学、分子动力学、体外和体内实验,探讨蟾毒素治疗胃癌的作用机制
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
蟾毒素是中国广泛使用的传统药物之一,是蟾素的主要生物活性成分之一,已被用于治疗包括胃癌在内的各种恶性肿瘤。然而,其对胃癌的抗肿瘤作用及其机制尚未完全阐明。本研究旨在从体外和体内两方面全面研究蟾毒素对AGS胃癌细胞的作用,并进一步探讨其作用机制。用蟾毒素处理AGS胃癌细胞。通过一系列的实验和分析,以及在体内的胃癌模型,研究了AGS细胞特征的变化,并阐明了蟾毒球蛋白作用的分子机制。我们的数据表明,cinobufagin在AGS胃癌细胞中表现出多种抗胃癌活性,包括抑制增殖、诱导凋亡、阻滞细胞周期于G0/G1期、抑制迁移和侵袭。此外,蛋白质组学分析、生物信息学分析、分子对接和分子动力学模拟表明,decorin (DCN)最可能是cinobufagin的靶点。Western blot和免疫组化(IHC)实验证实,cinobufagin在AGS细胞和异种移植物肿瘤中均显著上调DCN的表达。此外,蟾毒球蛋白能有效降低EGFR和活性EGFR水平,抑制Erk磷酸化,这可能与其抗胃癌作用有关。Cinobufagin可能通过DCN/EGFR途径抑制小鼠胃肿瘤生长,可能作为胃癌的潜在治疗选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
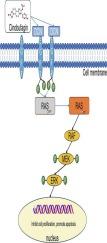
Exploring the mechanism of cinobufagin on gastric cancer based on proteomics, molecular dynamics, in vitro and in vivo experiment
Cinobufagin as one of the primary bioactive components of Chansu, which is a widely used traditional remedy in China, has been employed in the treatment of various malignant tumors, including gastric cancer. However, its antitumor effects on gastric cancer and the underlying mechanisms have not yet been fully elucidated. This study aims to comprehensively investigate the effects of cinobufagin on AGS gastric cancer cells both in vitro and in vivo, and to further explore its mechanism of action. AGS gastric cancer cells were treated with cinobufagin. A series of assays and analyses were conducted, and an in vivo gastric cancer model, to examine changes in AGS cell characteristics and to elucidate the molecular mechanisms underlying the effects of cinobufagin. Our data demonstrate that cinobufagin exhibits multiple anti-gastric cancer activities, including suppression of proliferation, induction of apoptosis, cell cycle arresting at the G0/G1 phase, and inhibition of migration and invasion in AGS gastric cancer cells. Furthermore, proteomic analysis, bioinformatics analysis, molecular docking, and molecular dynamics simulation suggest that decorin (DCN) is the most probable target of cinobufagin. Western blot and immunohistochemistry (IHC) assays confirmed that cinobufagin significantly upregulated DCN expression in both AGS cells and xenograft tumors. Moreover, cinobufagin effectively reduced the levels of EGFR and active EGFR, and inhibited Erk phosphorylation, which may contribute to its anti-gastric cancer effects. Cinobufagin is capable of suppressing gastric tumor growth in mice likely through the DCN/EGFR pathway and may serve as a potential treatment option for gastric cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


