局部应用磺酸,全氟丁烷磺酸(PFBS)和全氟戊烷磺酸(PFPeS)对小鼠模型的全身毒性。
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
全氟烷基和多氟烷基物质(PFAS)是一类因其化学和物理性质而加入产品中的合成表面活性剂。研究已将PFAS与不良健康影响联系起来。虽然皮肤接触的可能性很高,但缺乏与这种接触途径有关的毒性研究。本研究在小鼠模型中评估了皮肤亚慢性暴露于全氟丁烷磺酸(PFBS)(1.25-5 %)或全氟戊烷磺酸(PFPeS)(1.25-5 %)28天后的全身毒性。在血清和尿液中检测到两种PFAS水平升高,表明吸收是通过皮肤进行的。此外,两种PFAS均显著增加了相对肝脏重量,改变了血清化学成分,改变了皮肤和肝脏组织病理学,并显著降低了相对脾脏重量(仅PFPeS)。在肝脏和皮肤中观察到与脂肪酸代谢、炎症和皮肤完整性有关的基因表达变化。总的来说,与PFBS相比,pfps引起的终点变化更频繁,这支持了长链PFAS毒性更大的概念。这些发现支持PFAS通过皮肤吸收,导致肝脏损伤和全身毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
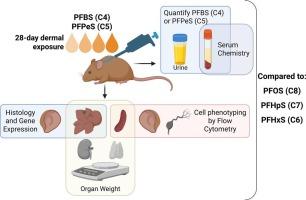
Systemic toxicity induced by topical application of the sulfonic acids, perfluorobutane sulfonic acid (PFBS) and perfluoropentane sulfonic acid (PFPeS), in a murine model
Per- and polyfluoroalkyl substances (PFAS) are a large group of synthetic surfactants incorporated into products for their chemical and physical properties. Studies have associated PFAS with adverse health effects. Although there is a high potential for dermal exposure, toxicity studies related to this route of exposure are lacking. The present study evaluated the systemic toxicity following a sub-chronic 28-day dermal exposure to perfluorobutane sulfonic acid (PFBS) (1.25–5 %) or perfluoropentane sulfonic acid (PFPeS) (1.25–5 %) in a murine model. Elevated levels of both PFAS were detected in the serum and urine, suggesting that absorption occurs through the skin. Additionally, both PFAS induced significantly increased relative liver weight, altered serum chemistries, altered skin and liver histopathology, and significantly decreased relative spleen weight (PFPeS only). Gene expression changes were observed in the liver and skin for genes involved in fatty acid metabolism, inflammation, and skin integrity. In general, the PFPeS-induced changes in the endpoints examined were observed more frequently compared to PFBS, supporting the concept that longer-chain PFAS are more toxic. These findings support PFAS absorption through the skin, leading to liver damage and systemic toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


