δ-MnO2室温催化降解乙烯及其在巫山李果实采后保鲜中的应用
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
采用异丙醇还原法(MnO2-iso)和氯化铵水热法(MnO2-NH4Cl)制备了两种用于室温乙烯催化降解的δ-MnO2催化剂。通过扫描电镜、x射线衍射和N2吸附-解吸分析等表征手段,探讨了其对乙烯降解差异的潜在机制。x射线光电子能谱分析表明,相对于MnO2-NH4Cl, MnO2-iso的性能增强主要源于其丰富的表面缺陷、Mn3+的增加和活性氧的增加。在100 ppm乙烯超过8 h时,MnO2-iso的降解效率为66.9% %,而MnO2-NH4Cl的降解效率为46.1% %。此外,将δ-MnO2加入到巫山梅果实采后处理的复合标签中,两种催化剂在第12天显著降低了乙烯产量(28.5% %和20.3% %),使呼吸峰值延迟2 d,更好地保存了果实的关键品质属性,延长了果实的保质期3-5 d。这些发现表明δ-MnO2是环境乙烯氧化在水果保存中的环保替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
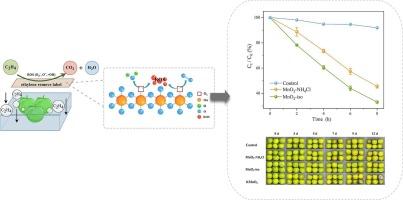
The catalytic degradation of ethylene by δ-MnO2 at room temperature and its application in the postharvest preservation of Prunus salicina ‘Wushan Plum’ fruit
This study developed two δ-MnO2 catalysts synthesized via isopropanol reduction (MnO2-iso) and ammonium chloride hydrothermal treatment (MnO2-NH4Cl) for room-temperature ethylene catalytic degradation. Characterizations, e.g., scanning electron microscope, X-ray diffraction and N2 adsorption-desorption analysis, are employed to investigate the underlying mechanism of their ethylene-degradation differences. X-ray photoelectron spectroscopy indicates that the enhanced performance of MnO2-iso primarily stems from its abundant surface defects, elevated Mn3+, and reactive oxygen species, relative to MnO2-NH4Cl. At 100 ppm ethylene over 8 h, MnO2-iso achieves 66.9 % degradation efficiency compared to 46.1 % for MnO2-NH4Cl. Furthermore, when incorporated into composite labels for postharvest treatment of Prunus salicina ‘Wushan Plum’ fruit, both catalysts significantly reduce ethylene production by (28.5 % and 20.3 % by 12th day), delay respiration peaks by 2 d, better preserve fruits' key quality attributes, and extend fruits' shelf life by 3–5 d. These findings highlight δ-MnO2 as an eco-friendly alternative for ambient ethylene oxidation in fruit preservation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


