膳食补充剂掺假:降低风险的实验室方法。
IF 3.6
2区 生物学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
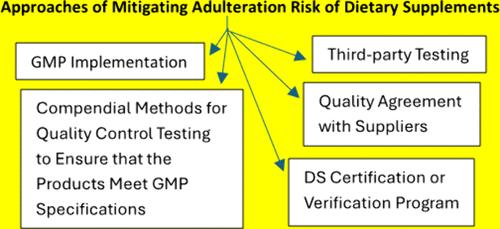
掺假问题是膳食补充剂(DS)制造商面临的一个持续问题。在过去几年中,主要的供应链中断进一步导致掺假风险增加。在美国销售产品的DS制造商必须遵守GMP,其中包括质量控制测试,以确保产品符合标识、纯度、强度和成分以及污染物限制的规格。除了使用药典方法进行质量控制测试外,帮助DS行业降低掺假风险的工具还包括DS认证或验证程序、与供应商达成的质量协议以及第三方测试。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dietary Supplement Adulteration: Laboratory Approaches to Risk Mitigation
Adulteration issues are a continuous problem facing dietary supplements (DS) manufacturers. In the past few years, major supply chain disruptions have further led to increased risk of adulteration. DS manufacturers selling products in the U.S. are required to comply with GMP, which include quality control testing to ensure that the products meet specifications for identity, purity, strength and composition, and limits on contaminants. In addition to quality control testing with compendial methods, tools to help the DS industry mitigate risks associated with adulteration include DS certification or verification programs, quality agreements with suppliers, and third-party testing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
5.90%
发文量
294
审稿时长
2.3 months
期刊介绍:
The Journal of Natural Products invites and publishes papers that make substantial and scholarly contributions to the area of natural products research. Contributions may relate to the chemistry and/or biochemistry of naturally occurring compounds or the biology of living systems from which they are obtained.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.
When new compounds are reported, manuscripts describing their biological activity are much preferred.
Specifically, there may be articles that describe secondary metabolites of microorganisms, including antibiotics and mycotoxins; physiologically active compounds from terrestrial and marine plants and animals; biochemical studies, including biosynthesis and microbiological transformations; fermentation and plant tissue culture; the isolation, structure elucidation, and chemical synthesis of novel compounds from nature; and the pharmacology of compounds of natural origin.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


