新形态蛋白界面催化肿瘤中RASG12D天冬氨酸的共价抑制
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
突变的RAS蛋白是人类癌症最普遍的驱动因素之一,密码子12 (G12D)上的甘氨酸到天冬氨酸突变是最常见的变体。突变选择性共价抑制剂在健康组织中保留RAS,并使其具有广泛的药理学作用,但共价靶向RASG12D受到低亲核性和高羧酸蛋白质组学丰富度的阻碍。我们通过结合亲环蛋白A (CYPA)的化合物克服了这些挑战,在CYPA和活性RAS之间建立了一种新形态蛋白-蛋白界面,使D12和亲电弹头之间的共价反应具有选择性,酶样速率增强,具有极低的固有反应活性。该方法获得了在多种KRASG12D癌症临床前模型中具有显著抗肿瘤活性的口服生物可利用化合物,包括正在进行临床评估的研究药物zoldonrasib (rmmc -9805) (NCT06040541)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
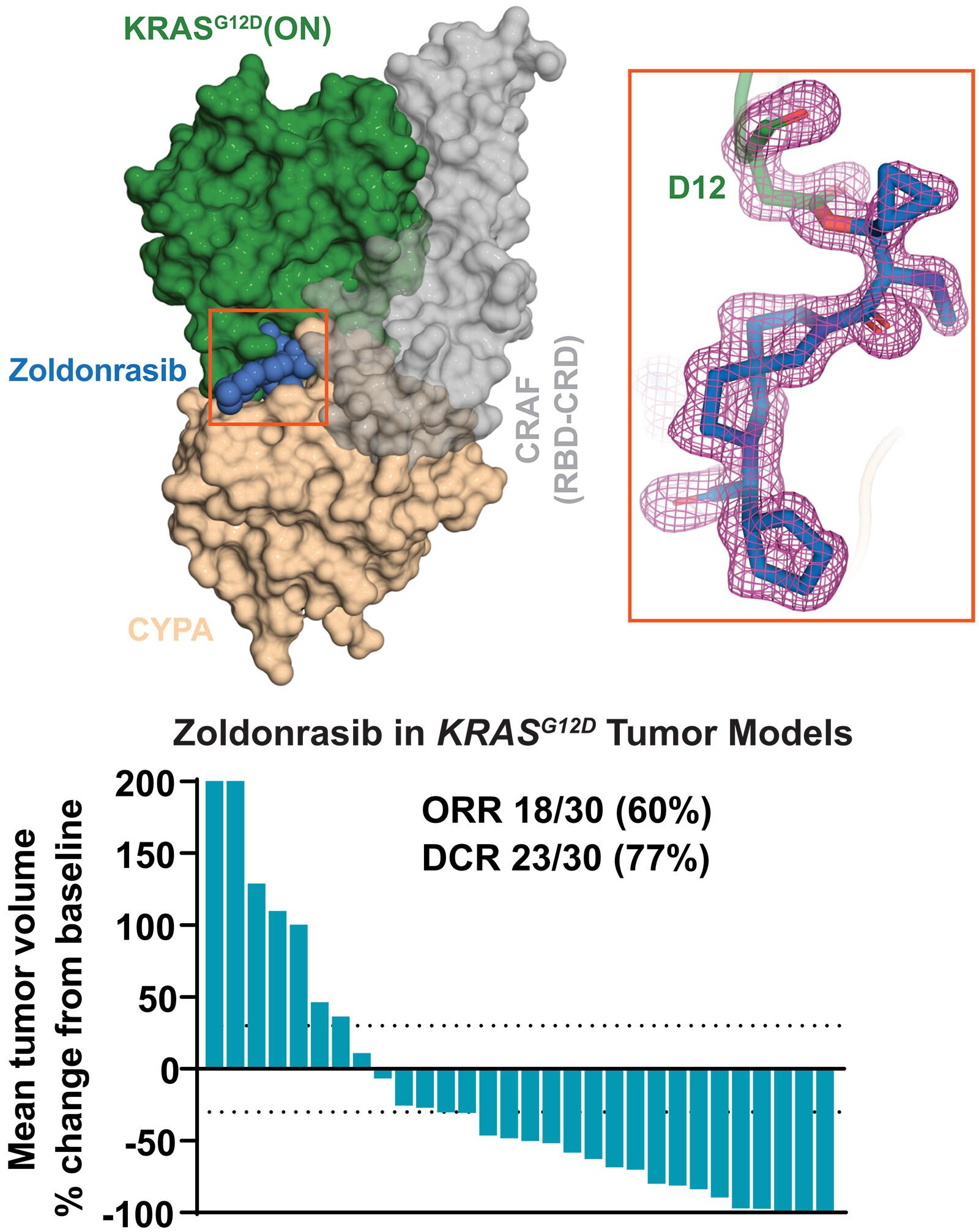
A neomorphic protein interface catalyzes covalent inhibition of RASG12D aspartic acid in tumors
Mutant RAS proteins are among the most prevalent drivers of human cancer, and the glycine to aspartic acid mutation at codon 12 (G12D) is the most common variant. Mutation-selective covalent inhibitors spare RAS in healthy tissue and enable extended pharmacodynamic effect, but covalent targeting of RASG12D is hindered by low nucleophilicity and high proteomic abundance of carboxylic acids. We overcame these challenges with compounds that bind cyclophilin A (CYPA) to create a neomorphic protein-protein interface between CYPA and active RAS that enables selective, enzyme-like rate enhancement of the covalent reaction between D12 and electrophilic warheads with exceptionally low intrinsic reactivity. This approach yielded orally bioavailable compounds with marked antitumor activity in multiple preclinical models of KRASG12D cancers, including the investigational agent zoldonrasib (RMC-9805) currently undergoing clinical evaluation (NCT06040541).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


