单次或多次咽部吸入二氧化钛纳米颗粒后的生物分布和毒性:最大允许剂量水平的测定。
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
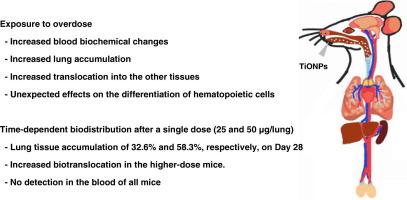
建立合理的暴露条件对于最大限度地发挥纳米材料的潜在效益,同时确保其安全性至关重要。我们首先确定了不同暴露情景(50 μg 4次,100 μg 2次,200 μg 1次)下过量暴露对肺组织损伤和沉积水平的影响。在接受200 μg剂量的组中,血液中IP和K的水平发生了显著变化。在所有治疗组中,肺组织中残留的TiONPs数量显著增加,同时肺细胞总数增加,巨噬细胞比例下降。在所有tionp处理组中均观察到I型辅助性T细胞主导的炎症反应,并且在暴露于单剂量200 μg的tionp的小鼠肺组织中,色素巨噬细胞的积累最为显著。然后,我们研究了单次剂量(25和50 μg/肺)后的生物分布和清除水平。随着时间的推移,残留在肺中的多肽的数量减少。然而,在给药25 μg和50 μg的小鼠肺中,第1天检测到的量分别约为32.6% %和58.3% %,持续到第28天。在暴露于最高剂量的小鼠中,转移到其他组织的现象更为明显,更有趣的是,在单次剂量后的第28天,转移到肝脏的TiONPs的清除率有限。综上所述,过量的TiONPs可能通过影响肺泡巨噬细胞的功能来促进组织积聚和易位。此外,考虑到高组织沉积率,我们建议应仔细确定呼吸道的最大允许剂量水平。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biodistribution and toxicity following a single or repeated pharyngeal aspiration of titanium dioxide nanoparticles: Determination of maximum allowable dose level
Establishing reasonable exposure conditions is crucial for maximizing the potential benefits of nanomaterials while ensuring their safety. We first identified the effects of overdose on lung tissue damage and deposition levels under different exposure scenarios (50 μg four times, 100 μg twice, and 200 μg once). Blood levels of IP and K changed significantly in the group that received a single 200 μg dose. In all TiONP-treated groups, the amount of TiONPs remaining in the lung tissues increased dramatically, accompanied by an increase in the total number of pulmonary cells and a decrease in the proportion of macrophages. A type I helper T cell-dominant inflammatory response was observed across all TiONP-treated groups, and the accumulation of pigmented macrophages was most notable in the lung tissue of mice exposed to a single dose of 200 μg of TiONPs. We then investigated biodistribution and clearance levels over time after a single dose (25 and 50 μg/lung). The amount of TiONPs remaining in the lung decreased over time. However, approximately32.6 % and 58.3 % of the amounts detected on Day 1 after a single dose persisted until Day 28 in the lungs of mice given 25 μg and 50 μg, respectively. Translocation to other tissues was more notable in mice exposed to the highest dose, and more interestingly, the TiONPs that moved to the liver showed limited clearance by Day 28 after a single dose. In conclusion, overdosed TiONPs may facilitate tissue accumulation and translocation by affecting the function of alveolar macrophages. Additionally, considering the high tissue deposition rate, we suggest that the maximum allowable dose level for the respiratory tract should be carefully determined.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.80
自引率
2.60%
发文量
309
审稿时长
32 days
期刊介绍:
Toxicology and Applied Pharmacology publishes original scientific research of relevance to animals or humans pertaining to the action of chemicals, drugs, or chemically-defined natural products.
Regular articles address mechanistic approaches to physiological, pharmacologic, biochemical, cellular, or molecular understanding of toxicologic/pathologic lesions and to methods used to describe these responses. Safety Science articles address outstanding state-of-the-art preclinical and human translational characterization of drug and chemical safety employing cutting-edge science. Highly significant Regulatory Safety Science articles will also be considered in this category. Papers concerned with alternatives to the use of experimental animals are encouraged.
Short articles report on high impact studies of broad interest to readers of TAAP that would benefit from rapid publication. These articles should contain no more than a combined total of four figures and tables. Authors should include in their cover letter the justification for consideration of their manuscript as a short article.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


