优化双功能策略下固定化磷脂酶D合成磷脂酰丝氨酸的Pickering界面生物催化
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
磷脂酰丝氨酸(PS)是一种在功能性食品和药品中具有很高价值的磷脂。然而,缺乏合适的生物催化系统限制了转化效率。本研究开发了一种新的皮克林界面生物催化(PIB)系统,用于将磷脂酰胆碱(PC)转化为PS。通过在中空介孔二氧化硅颗粒(HMSP)上接枝不同的修饰基团,合成了三种固定化酶载体。NQ-62与辛基修饰基团组合得到最佳固定化酶PLD@HMSP-N3/C8。它通过氢键作用稳定了油包水Pickering乳状液,提高了磷脂酶D (PLD)的稳定性。在优化条件下,在40 ℃条件下,PS转化率在20 min内达到93.8 %,催化效率(CE)为372 mmol/(g·h)。与游离和同源系列相比,固定化PLD表现出明显增强的热稳定性、耐pH性和蛋白酶水解稳定性。而且,在10次 循环使用后,它保留了70.9 %的PS转化率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
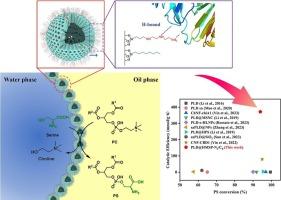
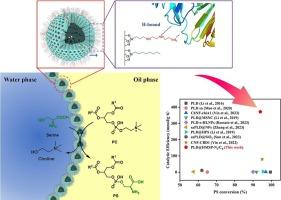
Enhanced Pickering interfacial biocatalysis in phosphatidylserine synthesis via phospholipase D immobilization with optimized bifunctional strategy
Phosphatidylserine (PS) is a high-value phospholipid in functional foods and pharmaceuticals. However, the lack of a suitable biocatalytic system has limited the conversion efficiency. A novel Pickering interfacial biocatalysis (PIB) system has developed for converting phosphatidylcholine (PC) to PS in this work. Three immobilized enzyme carriers were synthesized by grafting different modifying groups onto hollow mesoporous silica particles (HMSP). The combination of NQ-62 and octyl-modified groups yielded the optimal immobilized enzyme, PLD@HMSP-N3/C8. It stabilized the water-in-oil Pickering emulsion and enhanced phospholipase D (PLD) stability via hydrogen bond interactions. Under optimized conditions, a PS conversion of 93.8 % was achieved within 20 min at 40 °C, achieving a catalytic efficiency (CE) of 372 mmol/(g·h). The immobilized PLD demonstrated markedly enhanced thermostability, pH resistance, and protease hydrolysis stability compared to its free and homologous series counterparts. Moreover, it retained 70.9 % of the PS conversion after 10 cycles of reuse.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


