大豆分离蛋白原纤维与原花青素动态非共价键变化机制:pH调节途径
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本文主要研究了大豆分离蛋白原纤维(SPIF)与原花青素(OPC)通过pH调节途径发生非共价键变化的机制。OPC可使SPIF的荧光发生静态猝灭。在pH 4时,两者的自发结合以静电相互作用和疏水相互作用为主。相反,除了pH 4外,两者都通过氢键、范德华力和疏水相互作用自发结合。当pH值从2增加到7时,蛋白质的结构展开和重排发生,破坏了SPIF及其复合物β-片结构。在pH 5,接近蛋白质的等电点时,柔性结构显著降低,溶解性和乳化性降至最低。原纤维形态逐渐消失并聚集。OPC的加入显著提高了SPIF的乳化和发泡活性。此外,在pH 4时,OPC的加入最大程度地改善了SPIF的功能性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
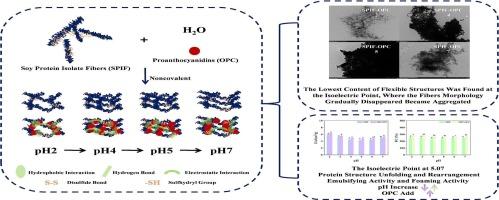
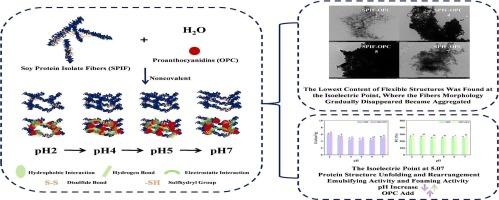
Mechanism of dynamic noncovalent bonding changes between soy protein isolate fibrils and proanthocyanidins: A pathway for pH tunning
This paper focused on the mechanism of dynamic noncovalent bonding changes through a pathway for pH tunning between soy protein isolate fibrils (SPIF) and proanthocyanidins (OPC). OPC could produce a static quenching on the fluorescence of SPIF. At pH 4, the spontaneous binding of the two was dominated by electrostatic interaction and hydrophobic interaction. In contrast, except at pH 4, both bound spontaneously through hydrogen bonding, van der Waals force and hydrophobic interaction. With an increase in pH from 2 to 7, structural unfolding and rearrangement of the protein occurred, disrupting the SPIF and its complexes β-sheet structure. At pH 5, close to the isoelectric point of protein, flexible structures were significantly reduced, and solubility and emulsifying properties were minimized. Fibrils morphology gradually disappeared and became aggregated. The SPIF emulsifying and foaming activity were significantly improved with the addition of OPC. In addition, the SPIF functional properties were improved to the greatest extent at pH 4 with the addition of OPC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


