性别连锁染色体倒位的渗入动力学形成马拉维慈鲷辐射
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science
Pub Date : 2025-06-12
引用次数: 0
摘要
染色体倒位可以通过连接共适应等位基因来促进适应性物种形成。通过查询1375个物种丰富的马拉维慈鲷鱼辐射的基因组,我们发现了底栖亚辐射中分离的五个大反转,每个反转抑制了超过一半的染色体重组。其中两个反转来自深海梁龙,而其他反转则建立在深海底栖进化支的早期。马拉维辐射内外的单倍型谱系的渗入与物种多样化的爆发相一致。倒位显示了短暂性联系的证据,并且显著过量的蛋白质改变替代指向神经感觉、生理和生殖基因的选择。这些结果表明,深度适应和性别特异性选择之间的反复相互作用是这一标志性系统进化的核心。本文章由计算机程序翻译,如有差异,请以英文原文为准。
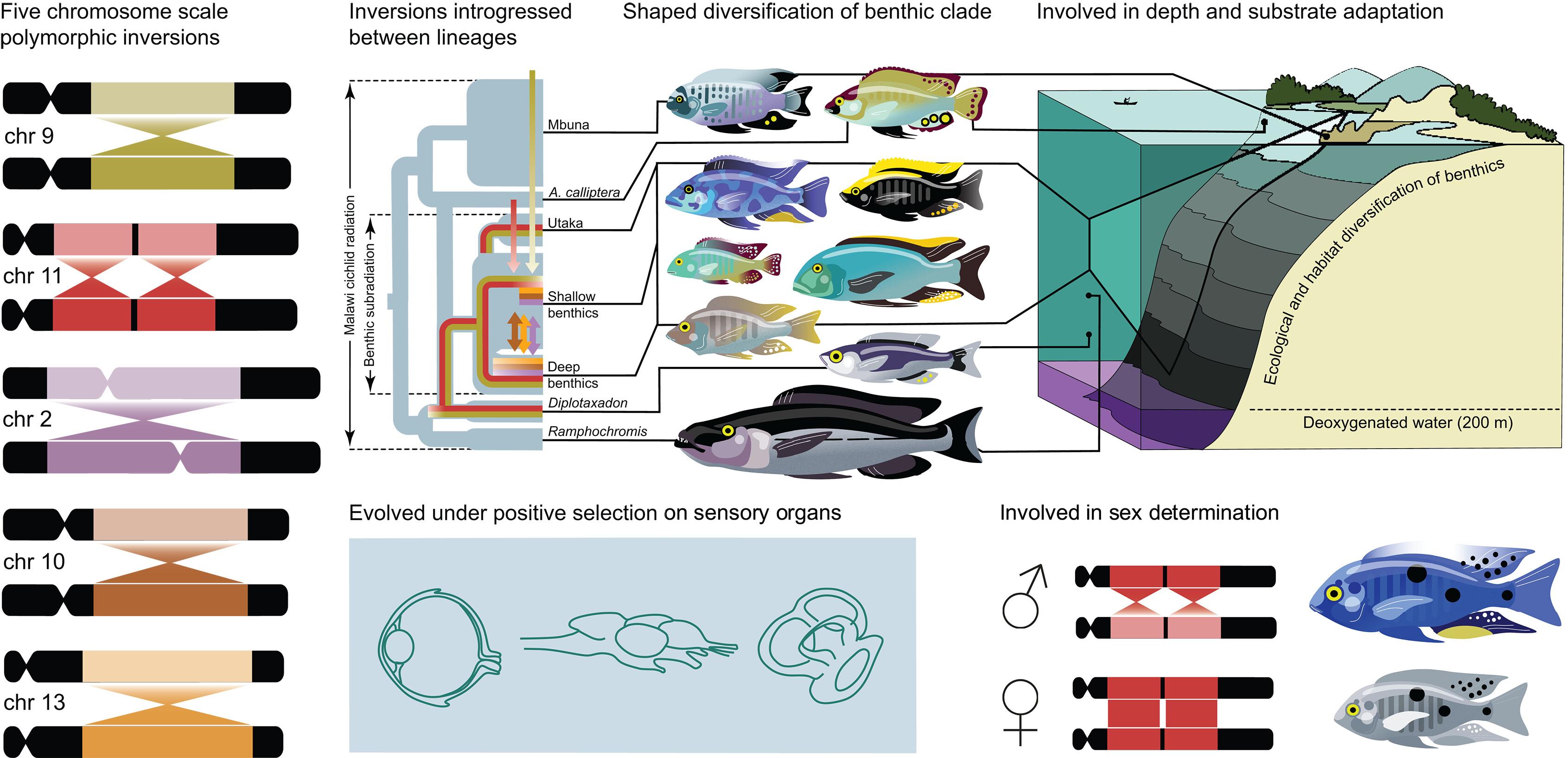
Introgression dynamics of sex-linked chromosomal inversions shape the Malawi cichlid radiation
Chromosomal inversions can contribute to adaptive speciation by linking coadapted alleles. By querying 1375 genomes of the species-rich Malawi cichlid fish radiation, we discovered five large inversions segregating in the benthic subradiation that each suppress recombination over more than half a chromosome. Two inversions were transferred from deepwater pelagic Diplotaxodon through admixture, whereas the others established early in the deep benthic clade. Introgression of haplotypes from lineages inside and outside the Malawi radiation coincided with bursts of species diversification. Inversions show evidence for transient sex linkage, and a notable excess of protein changing substitutions points toward selection on neurosensory, physiological, and reproductive genes. These results indicate that repeated interplay between depth adaptation and sex-specific selection on large inversions has been central to the evolution of this iconic system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


