酶催化-溶出增强时间分辨荧光免疫分析的级联信号放大
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
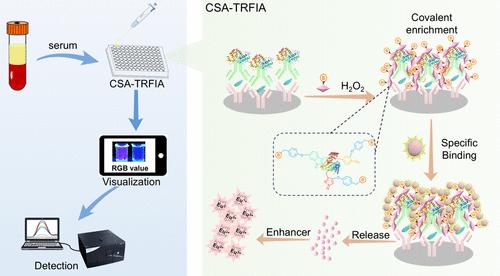
时间分辨荧光免疫分析法(TRFIA)因其低背景噪声的特点在各个领域得到了广泛的应用。然而,镧系离子复合物在抗体上的有限附着限制了信号强度,使得检测低丰度的分析物具有挑战性。为了克服这一挑战,我们开发了一种级联信号放大策略,将酶催化酪胺信号放大(TSA)与氟化镧纳米探针(NPs)的溶解增强相结合。在传统的酶联免疫吸附试验(ELISA)中,TSA过程(初级信号扩增)丰富了免疫复合物周围的生物素部分,然后附着链霉亲和素修饰的NaEuF4 NPs。当暴露于酸性条件下,NaEuF4 NPs解离成大量的Eu3+离子。然后,每个Eu3+离子可以通过与增强剂的螯合形成发射镧系离子络合物,导致二次信号放大。作为概念验证,该方法用于检测抗本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cascade Signal Amplification of Time-Resolved Fluorescence Immunoassay through Enzyme Catalysis Coupled with Dissolution Enhancement
Time-resolved fluorescence immunoassay (TRFIA) has found wide application in various fields due to its low background noise characteristics. However, the limited attachment of lanthanide ion complexes onto antibodies restricts the signal intensities, making it challenging to detect analytes with low abundance. To overcome this challenge, we developed a cascade signal amplification strategy that combines enzyme-catalyzed tyramine signal amplification (TSA) with the dissolution enhancement of lanthanide fluoride nanoprobes (NPs). The TSA process (primary signal amplification) enriches biotin moieties around the immunocomplex in a conventional enzyme-linked immunosorbent assay (ELISA), followed by the attachment of streptavidin-modified NaEuF4 NPs. When exposed to acidic conditions, NaEuF4 NPs dissociate into a large quantity of Eu3+ ions. Each Eu3+ ion can then form an emissive lanthanide ion complex by chelation with enhancer agents, leading to secondary signal amplification. As a proof-of-concept, this approach is used to detect anti-Müllerian hormone (AMH), achieving a detection limit of 8.6 × 10–13 g/mL, which is superior to commercially available kits. The proposed TRFIA with cascade signal amplification benefits from the low background noise of TRFIA, simultaneously harnessing enhanced signals through dual amplification. This approach shows great potentials in improving the sensitivity of immunoassays and also achieving dual-mode detection capabilities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


