GPR45调节室旁下丘脑初级纤毛上的Gαs,控制食物摄入
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
黑素皮质素系统集中调节能量稳态,关键成分如黑素皮质素-4受体(MC4R)和腺苷酸环化酶3 (ADCY3)在神经元初级纤毛。MC4R和ADCY3的突变以及纤毛功能障碍导致肥胖,但黑素皮质素信号如何在纤毛中起作用尚不清楚。通过小鼠随机种系诱变,我们发现了G蛋白偶联受体45 (Gpr45)的两个错义突变,这些突变通过嗜食导致肥胖。GPR45表达于下丘脑室旁核(PVH),定位于纤毛,通过ADCY3募集Gαs增加纤毛环磷酸腺苷(cAMP)。GPR45在PVH纤毛中与MC4R共定位,促进纤毛MC4R的激活。PVH或MC4R+神经元中GPR45的缺失导致肥胖。这些发现表明GPR45是纤毛黑素皮质素系统的关键调节因子,连接MC4R和ADCY3。本文章由计算机程序翻译,如有差异,请以英文原文为准。
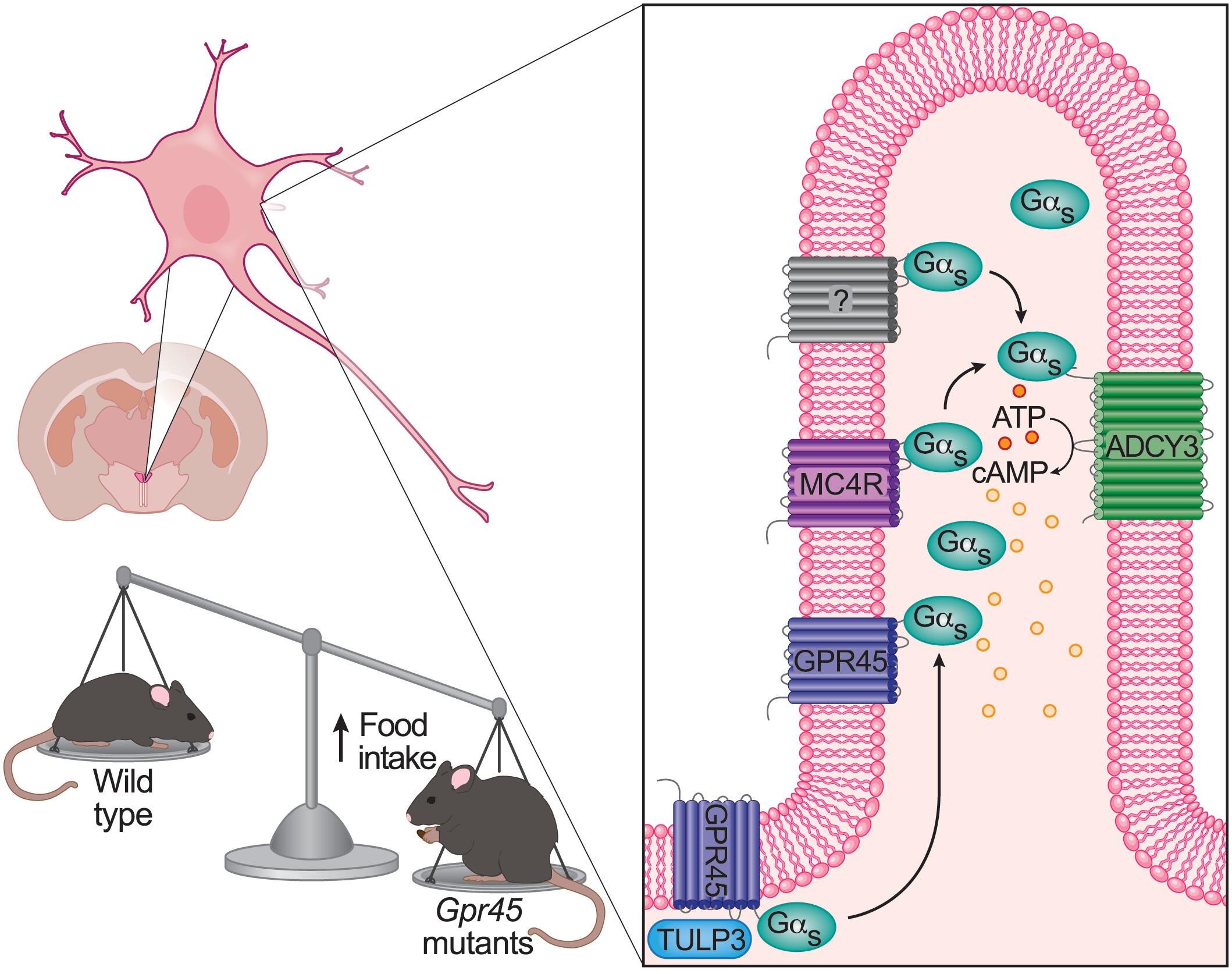
GPR45 modulates Gαs at primary cilia of the paraventricular hypothalamus to control food intake
The melanocortin system centrally regulates energy homeostasis, with key components such as melanocortin-4 receptor (MC4R) and adenylyl cyclase 3 (ADCY3) in neuronal primary cilia. Mutations in MC4R and ADCY3 as well as ciliary dysfunction lead to obesity, but how melanocortin signaling works in cilia remains unclear. Using mouse random germline mutagenesis, we identified two missense mutations in G protein–coupled receptor 45 (Gpr45) that lead to obesity through hyperphagia. GPR45 was expressed in paraventricular nucleus of the hypothalamus (PVH), where it localized to cilia and recruited Gαs to increase ciliary cyclic adenosine monophosphate (cAMP) via ADCY3. GPR45 colocalized with MC4R in PVH cilia and promoted ciliary MC4R activation. Loss of GPR45 in the PVH or MC4R+ neurons caused obesity. These findings establish GPR45 as a key regulator of the ciliary melanocortin system, bridging MC4R and ADCY3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


