用设计的远红多巴胺传感器进行动态神经化学网络的体内多重成像
IF 45.8
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
多巴胺(DA)通过与其他神经调节剂和细胞内信号通路的复杂相互作用,在多种脑功能中起着至关重要的作用。然而,对这些复杂网络的研究一直受到同时检测体内多种神经化学物质的挑战的阻碍。为了克服这一限制,我们开发了一种单蛋白化学DA传感器HaloDA1.0,它结合了cphalotag -化学染料方法和基于G蛋白偶联受体激活(GRAB)的策略,为DA提供了高灵敏度、亚秒级响应动力学和远红到近红外光谱范围。当HaloDA1.0与现有的绿色和红色荧光神经调节剂传感器、钙指示剂、环腺苷5 ' -单磷酸传感器和光遗传学工具一起使用时,HaloDA1.0在培养神经元、脑切片和行为动物的多重成像中显示出高度的通通性,有助于深入研究动态神经化学网络。本文章由计算机程序翻译,如有差异,请以英文原文为准。
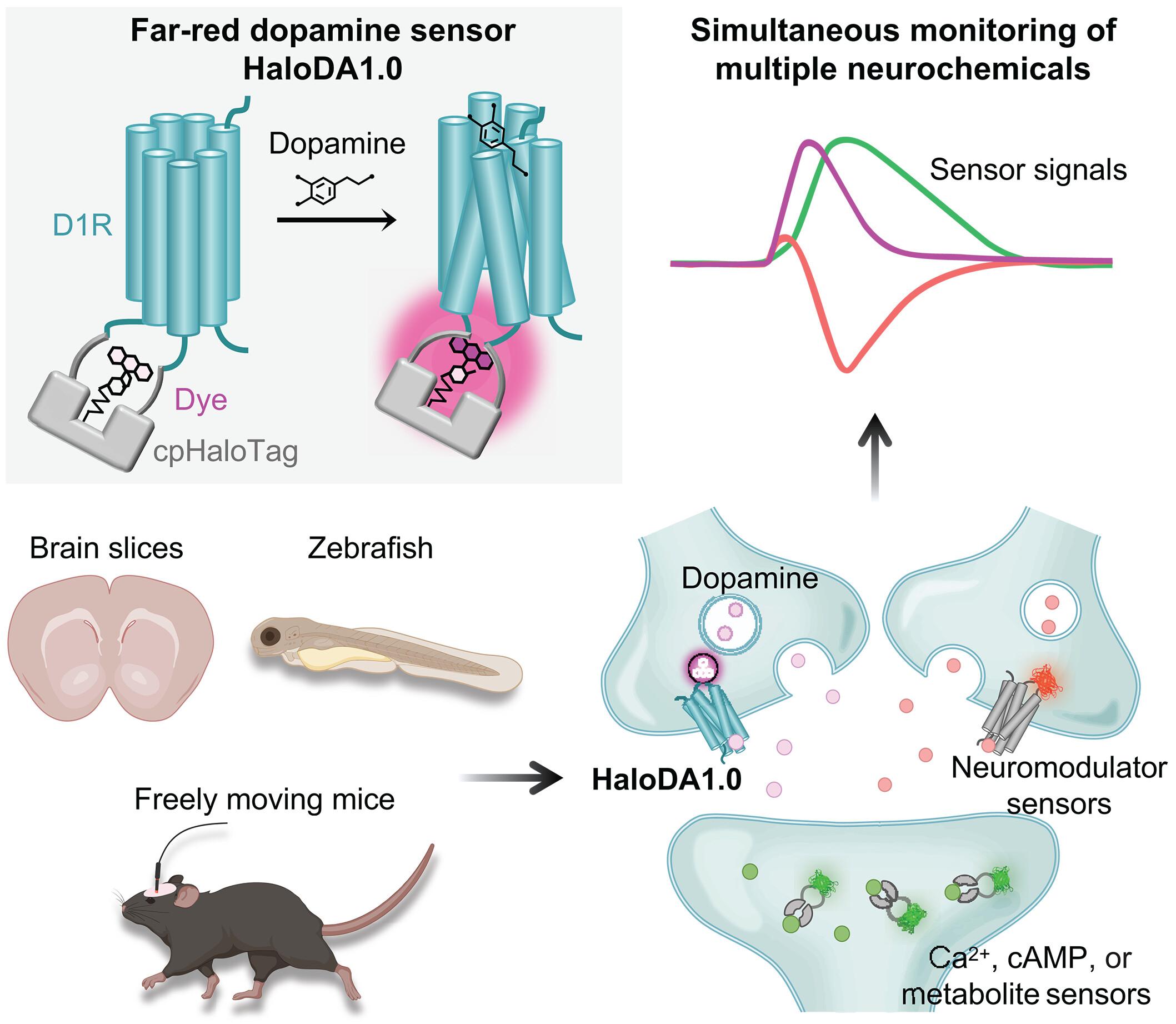
In vivo multiplex imaging of dynamic neurochemical networks with designed far-red dopamine sensors
Dopamine (DA) plays a crucial role in a variety of brain functions through intricate interactions with other neuromodulators and intracellular signaling pathways. However, studying these complex networks has been hindered by the challenge of detecting multiple neurochemicals in vivo simultaneously. To overcome this limitation, we developed a single-protein chemigenetic DA sensor, HaloDA1.0, which combines a cpHaloTag–chemical dye approach with the G protein–coupled receptor activation–based (GRAB) strategy, providing high sensitivity for DA, subsecond response kinetics, and a far-red to near-infrared spectral range. When used together with existing green and red fluorescent neuromodulator sensors, calcium indicators, cyclic adenosine 5′-monophosphate sensors, and optogenetic tools, HaloDA1.0 showed high versatility for multiplex imaging in cultured neurons, brain slices, and behaving animals, facilitating in-depth studies of dynamic neurochemical networks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science
综合性期刊-综合性期刊
CiteScore
61.10
自引率
0.90%
发文量
0
审稿时长
2.1 months
期刊介绍:
Science is a leading outlet for scientific news, commentary, and cutting-edge research. Through its print and online incarnations, Science reaches an estimated worldwide readership of more than one million. Science’s authorship is global too, and its articles consistently rank among the world's most cited research.
Science serves as a forum for discussion of important issues related to the advancement of science by publishing material on which a consensus has been reached as well as including the presentation of minority or conflicting points of view. Accordingly, all articles published in Science—including editorials, news and comment, and book reviews—are signed and reflect the individual views of the authors and not official points of view adopted by AAAS or the institutions with which the authors are affiliated.
Science seeks to publish those papers that are most influential in their fields or across fields and that will significantly advance scientific understanding. Selected papers should present novel and broadly important data, syntheses, or concepts. They should merit recognition by the wider scientific community and general public provided by publication in Science, beyond that provided by specialty journals. Science welcomes submissions from all fields of science and from any source. The editors are committed to the prompt evaluation and publication of submitted papers while upholding high standards that support reproducibility of published research. Science is published weekly; selected papers are published online ahead of print.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


