含有1,2,3-三唑基团的百里香酚衍生物的设计与合成及其对木瓜抗枯萎病的保护作用
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
唑类杀菌剂是市场上使用最广泛和最有效的杀菌剂之一。然而,它们的滥用会导致药效降低和病原体耐药性增加。这突出了对新型杀菌剂的需求,这种杀菌剂可以提高效率,降低对植物病原真菌的环境影响。本研究以铜(I)催化叠氮-炔环加成(CuAAC)反应为关键步骤,通过三步合成了一系列含有1,2,3-三唑基团的新型百里香酚衍生物20个。研究了这些化合物对番木瓜果实和茎腐病病原菌茄枯菌(Fusarium solani)的抑菌活性,并对其与茄枯菌(F. solani lanosterol 14α-demethylase, FsCYP51)酶的结合能和相互作用模式进行了分子对接。对接结果表明,与唑类杀菌剂四苯康唑(−8.2 kcal/mol)和底物毛甾醇(−9.0 kcal/mol)相比,FsCYP51的所有衍生物结合在催化口袋上的结合能均较低(−10 kcal/mol)。观察到的杀真菌活性可能是由于这些衍生物占据了FsCYP51的入口通道和活性位点,从而阻断了羊毛甾醇向麦角甾醇的转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
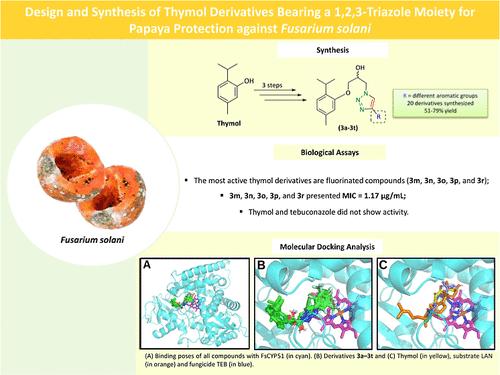
Design and Synthesis of Thymol Derivatives Bearing a 1,2,3-Triazole Moiety for Papaya Protection against Fusarium solani
Azole-based fungicides are among the market’s most widely used and effective agents. However, their indiscriminate use can lead to reduced efficacy and increased pathogen resistance. This highlights the need for novel fungicides that offer improved efficiency and lower environmental impact for controlling phytopathogenic fungi. In this study, a series of 20 novel thymol derivatives, incorporating a 1,2,3-triazole moiety, were synthesized via a three-step process, with the key step being the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction. The antifungal activity of these compounds was evaluated against Fusarium solani, the etiological agent of papaya fruit and stem rot. Additionally, molecular docking was performed to assess the binding energy and interaction modes of these derivatives with the F. solani lanosterol 14α-demethylase (FsCYP51) enzyme. Docking results demonstrated that all derivatives bound to the catalytic pocket of FsCYP51 with lower binding energy (<−10 kcal/mol) compared to the azole fungicide tebuconazole (−8.2 kcal/mol) and the substrate lanosterol (−9.0 kcal/mol). The observed fungicidal activity is likely due to the occupancy of the entrance tunnel and active site of the FsCYP51 by these derivatives, thereby blocking lanosterol and its conversion into ergosterol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


