新型β- n -乙酰氨基葡萄糖酶驱动晶体α-几丁质高效生物转化为n -乙酰- d -氨基葡萄糖及其促进机制分析
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
晶体α-几丁质的全酶级联降解是一个长期的研究课题,但一直受到转化效率低和产物多相的限制。本文对一种新型的从海洋硫roseicoccus oceanibius中提取的β- n -乙酰氨基葡萄糖苷酶(NAGase) Sohex进行了生物化学表征。该酶对n -乙酰基壳寡聚物(n -乙酰基COSs)具有较高的水解活性,对α-几丁质晶体降解具有促进作用。此外,基于多糖单加氧酶(LPMOs)、几丁质酶和Sohex的全酶级联降解体系被开发用于生产n -乙酰- d -葡萄糖胺(GlcNAc)。该体系转化率最高可达45.93%,GlcNAc纯度可达99.99%。Sohex显著提高了α-几丁质降解效率,与LPMOs +几丁质酶体系和单一几丁质酶体系相比,α-几丁质转化率分别提高了1.37倍和2.46倍。此外,还阐明了Sohex与LPMOs和几丁质酶结合的促进机制,表明级联系统是将结晶α-几丁质转化为高纯度GlcNAc的一种有前景的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
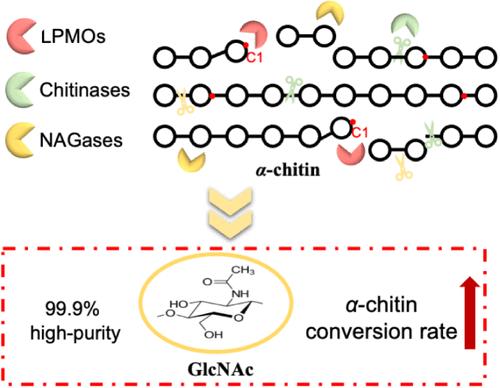
Efficient Bioconversion of Crystalline α-Chitin to N-Acetyl-D-glucosamine Driven by a Novel β-N-Acetylglucosaminidase and the Promotion Mechanism Analysis
Whole-enzyme cascade degradation of crystalline α-chitin is a long-term pursuit, which has been hampered by low transformation efficiency and heterogeneous products. Here, a novel β-N-acetylglucosaminidase (NAGase) from Sulfuriroseicoccus oceanibius, designated Sohex, was biochemically characterized. The enzyme demonstrated high hydrolytic activity toward N-acetyl chitooligomers (N-acetyl COSs) and exhibited promotive effects on crystalline α-chitin degradation. Furthermore, a whole-enzyme cascade degradation system based on lytic polysaccharide monooxygenases (LPMOs), chitinases, and Sohex was developed for N-acetyl-D-glucosamine (GlcNAc) production. This system achieved a maximum conversion rate of 45.93%, with GlcNAc purity reaching 99.99%. The addition of Sohex significantly enhanced α-chitin degradation efficiency, achieving conversion rates of 1.37-fold and 2.46-fold compared to the LPMOs + chitinases system and the sole chitinases system, respectively. Moreover, the promotion mechanisms of Sohex in conjunction with LPMOs and chitinases were elucidated, making the cascade system a promising strategy for converting crystalline α-chitin into high-purity GlcNAc.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


