开放路径光谱法检测羟基自由基:宽带腔增强吸收光谱(BBCEAS)与BBCEAS耦合法布里-普氏干涉仪(BBCEAS- fp)的比较
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
羟基自由基(OH)是主要的大气氧化剂,但由于其在大气条件下的半衰期短(约1秒)和低环境浓度(约106分子/cm3),测量起来很有挑战性。虽然存在几种测量技术,包括激光诱导荧光与气体膨胀荧光分析(liff - fage)、微分光学吸收光谱和化学电离质谱(CIMS),但通常只有liff - fage和CIMS被认为可用于现场测量。本研究比较了两种仪器的性能:耦合CCD的宽带腔增强吸收光谱仪(BBCEAS)和耦合法布里-普氏干涉仪(BBCEAS- fp)。半便携式BBCEAS仪器具有光谱特异性,避免了其他物种的干扰,并具有固有校准功能,消除了校准误差。还评估了气溶胶和湍流对性能的影响。采用低损耗光学器件测量开路结构下的光腔反射率,测试时使用丁烷火焰作为OH源。BBCEAS仪器对常温OH的1小时检出限(1σ)为1.5 × 107分子/cm3。应用高斯-埃尔米特滤波器将消光光谱中的噪声降低了2.66倍,将外推的检测限降低到4.6 × 106分子/cm3。bbceasl - fp是一种更具成本效益的便携式仪器,其外推1-h (1σ)检测限为1.5 × 107个分子/cm3。本文章由计算机程序翻译,如有差异,请以英文原文为准。
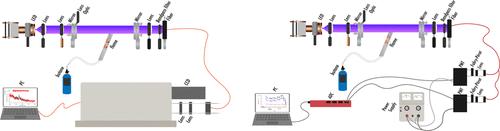
Open Path Spectroscopic Detection of Hydroxyl Radical: A Comparison between Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) and BBCEAS Coupled with a Fabry–Pérot Interferometer (BBCEAS-FP)
Hydroxyl radical (OH) is the primary atmospheric oxidant, but it is challenging to measure due to its short half-life under atmospheric conditions (approximately 1 s) and low ambient concentrations (approximately 106 molecules/cm3). While several measurement techniques exist, including laser-induced fluorescence with fluorescence assay by gas expansion (LIF-FAGE), differential optical absorption spectroscopy, and chemical ionization mass spectrometry (CIMS), often only LIF-FAGE and CIMS are considered available for field measurements. This study compares the performance of two instruments: a broadband cavity-enhanced absorption spectroscopy (BBCEAS) coupled with a CCD and a BBCEAS coupled with a Fabry–Pérot interferometer (BBCEAS-FP). The semiportable BBCEAS instrument benefits from spectroscopic specificity, avoiding interference from other species, and features inherent calibration, eliminating calibration errors. The effects of aerosols and turbulence on performance were also evaluated. A low-loss optic was used to measure the optical cavity reflectivity in an open-path configuration, and a butane flame served as the OH source during testing. The BBCEAS instrument achieved an extrapolated 1-h detection limit (1σ) of 1.5 × 107 molecules/cm3 for ambient-temperature OH. Applying a Gauss–Hermite filter reduced noise in the extinction spectrum by 2.66 times, lowering the extrapolated detection limit to 4.6 × 106 molecules/cm3. The BBCEAS-FP, a more cost-effective and portable instrument, demonstrated a comparable extrapolated 1-h (1σ) detection limit of 1.5 × 107 molecules/cm3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


