用铁化合物强化咖啡,以提高其微量营养成分
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究评估了不同形式的铁,硫酸亚铁(FS)、葡萄糖酸亚铁(FG)、EDTA铁钠(FSE)、焦磷酸铁(FPP)、富马酸亚铁(FF)、双甘氨酸亚铁(FB)和电解铁(EI)用于强化Arabica咖啡。fse强化咖啡(FC)表现出最高的溶解度和稳定性,铁回收率为4.702 mg / 100 ml,其次是FB-FC(3.681 mg)、FS-FC(3.345 mg)和FG-FC(3.435 mg)。ATR-FTIR证实了FSE和咖啡多酚之间最小的相互作用,确保了高保留和低络合(<1.0 mmol FeCl₃/TAE)。感官分析将FSE-FC与理想的香气、风味和口感联系起来,而EI-FC和FF-FC则表现出难闻的味道。GC-MS谱显示对关键挥发性化合物的影响最小。这些发现证实,FSE,其次是FB、FS和FG,是咖啡中最有效的铁强化剂,同时保持了咖啡的感官和物理化学特性,从而增强了咖啡作为一种促进公众健康的功能性食品的地位。本文章由计算机程序翻译,如有差异,请以英文原文为准。
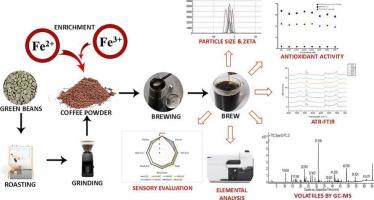
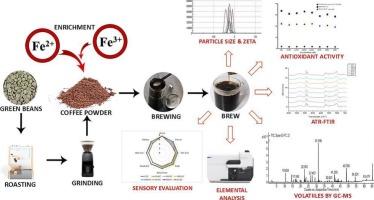
Fortification of coffee with iron compounds to enhance its micronutrient profile
This study evaluated the various iron forms, ferrous sulfate (FS), ferrous gluconate (FG), ferric sodium EDTA (FSE), ferric pyrophosphate (FPP), ferrous fumarate (FF), ferrous bis-glycinate (FB), and electrolytic iron (EI) for fortifying Arabica coffee. FSE-fortified coffee (FC) exhibited the highest solubility and stability, with an iron recovery of 4.702 mg per 100 mL, followed by FB-FC (3.681 mg), FS-FC (3.345 mg), and FG-FC (3.435 mg). ATR-FTIR confirmed minimal interaction between FSE and coffee polyphenols, ensuring high retention and low complexation (<1.0 mmol FeCl₃/TAE). Sensory analysis linked FSE-FC to desirable aroma, flavor, and mouthfeel, whereas EI-FC and FF-FC exhibited off-flavors. GC–MS profile indicated a minimal impact on key volatile compounds. These findings confirm that FSE, followed by FB, FS, and FG, are the most effective iron fortificants for coffee while preserving sensory and physicochemical properties, thereby enhancing coffee as a functional food that promotes better public health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


