基于非极性氨基酸扫描的高效诱变策略提高β-半乳糖苷酶的转糖基化能力
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
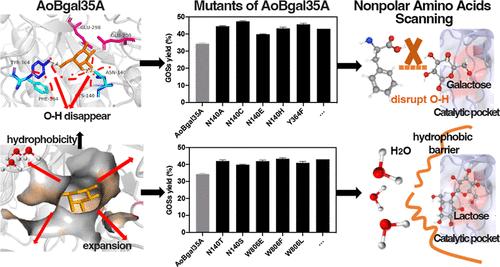
采用半设计方法对米曲霉β-半乳糖苷酶进行了基因修饰,提高了其转糖基化生产低半乳糖(GOSs)的能力。通过破坏氢键、改变疏水性和扩大催化袋,获得了12个单点突变体和一个具有增强的跨半乳糖基化能力的组合突变体(M3)。突变体M3在黑曲霉中成功表达,β-半乳糖苷酶产量达到228.2 U/mL。M3高效催化合成GOS,产率高达62.3% (w/w),与报道的GOS产率最高值(63.3%,w/w)相当。结构分析表明,AoBgal35A较弱的酶-半乳糖相互作用和较高的疏水性可能是AoBgal35A跨半乳糖基化能力增强的原因。因此,在调节酶-半乳糖相互作用和催化袋的疏水性的基础上,构建了非极性氨基酸扫描诱变策略。为了验证该策略,对3个β-半乳糖苷酶进行进一步修饰,其中2个酶的GOS产率提高了30-40%。该研究为GOS的商业化生产提供了良好的催化剂,也为β-半乳糖苷酶的快速修饰提供了策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficient Mutagenesis Strategy Based on Nonpolar Amino Acids Scanning for the Improvement of Transglycosylation Ability of β-Galactosidases
A commercial β-galactosidase from Aspergillus oryzae was genetically modified through semirational design to enhance its transglycosylation ability for galactooligosaccharides (GOSs) production. By disrupting hydrogen bonds, altering the hydrophobicity and enlarging the catalytic pocket, 12 single-point mutants and a combinatorial mutant (M3) with enhanced transgalactosylation abilities were obtained. Mutant M3 was successfully expressed in Aspergillus niger, and a β-galactosidase production of 228.2 U/mL was achieved. M3 efficiently catalyzed the synthesis of GOSs, with a high yield of 62.3% (w/w), which was comparable to that of the highest value for GOS production (63.3%, w/w) ever reported. Structural analysis revealed that weak enzyme-galactose interaction and high hydrophobicity of the catalytic pocket may contribute to the enhancement of transgalactosylation ability of AoBgal35A. Thus, a mutagenesis strategy named nonpolar amino acids scanning was constructed on the basis of adjusting enzyme-galactose interaction as well as the hydrophobicity of the catalytic pocket. To validate the strategy, 3 β-galactosidases were further modified and the GOS yields of 2 were improved by 30–40%. This study may provide an excellent catalyst for commercial GOS production as well as a rapid strategy for the modification of β-galactosidases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


