探讨豌豆分离蛋白-芦丁配合物的结合机理及动态变化,有效提高豌豆分离蛋白的发泡性能
IF 9.8
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
扩大豌豆分离蛋白(PPI)-芦丁(Ru)复合物在充气食品中的应用需要对其相互作用的分子机制有全面的了解。本研究采用多光谱技术、分子对接、分子动力学模拟等方法,发现PPI与Ru的结合机制涉及静态猝灭。色氨酸疏水微环境的改变也得到了证实。驱动PPI与Ru相互作用的主要因素是疏水相互作用,其次是氢键相互作用和静电相互作用。对PPI和Ru结合贡献最大的是59ASN、60LYS和63ARG,它们的结合促进了PPI二级结构的部分展开。Ru浓度为0.1024 mM时,PPI的结构柔韧性和表面疏水性得到有效改善。所制得的泡沫具有气/水界面膜厚、尺寸小、排列致密等特点,发泡性能最佳。本文章由计算机程序翻译,如有差异,请以英文原文为准。
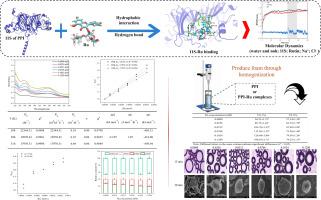
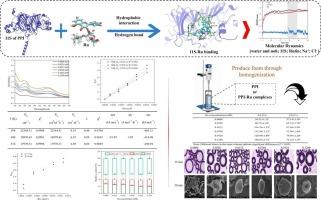
Explore the binding mechanism and dynamic variation of pea protein isolate-rutin complexes to effectively improve the foam performance of pea protein isolate
Expanding the utilization of pea protein isolate (PPI)-rutin (Ru) complexes in aerated foods requires a comprehensive understanding of the molecular mechanisms governing their interactions. This study was conducted using multi-spectroscopic techniques, molecular docking, and molecular dynamics simulations and found that the binding mechanism of PPI with Ru involved static quenching. A change in the hydrophobic microenvironment of tryptophan was also confirmed. The primary factors driving the interaction between PPI and Ru were hydrophobic interactions, followed by hydrogen bonding and electrostatic interaction. The most significant contributors to the binding of PPI and Ru were 59ASN, 60LYS, and 63ARG, and their binding promoted the partial unfolding of the secondary structure of PPI. The structural flexibility and surface hydrophobicity of PPI were effectively improved when Ru concentration was 0.1024 mM. The produced foam was characterized by a thicker air/water interfacial film, smaller size, and denser arrangement, resulting in the best foaming properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Chemistry
工程技术-食品科技
CiteScore
16.30
自引率
10.20%
发文量
3130
审稿时长
122 days
期刊介绍:
Food Chemistry publishes original research papers dealing with the advancement of the chemistry and biochemistry of foods or the analytical methods/ approach used. All papers should focus on the novelty of the research carried out.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


