分子光开关的反异构化动力学:液相色谱和离子迁移率测量的补充见解
IF 6.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
通过紫外线-可见光吸收分子的可逆异构化将太阳能储存在化学键中,为能量储存提供了一种前景广阔的方法。这些分子会形成高能光异构体,如果在动力学上有明显的活化屏障保护,防止自发的热反向异构化,就可以储存能量。在本研究中,我们比较了溶液中偶氮苯光电开关模型的反异构化动力学参数(ΔH‡ 和 ΔS‡)与使用原始串联离子迁移质谱仪在气相中获得的参数。我们的研究结果表明,从溶液相到气相,活化焓得到了很好的恢复,而活化熵则在没有溶剂的情况下受到很大影响,这进一步揭示了不同的弛豫机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
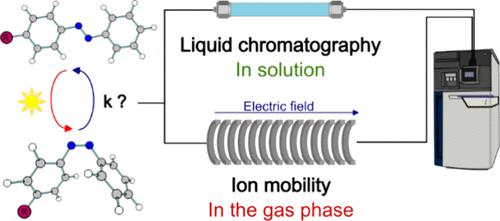
Back Isomerization Kinetics of Molecular Photoswitches: Complementary Insights from Liquid Chromatography and Ion Mobility Measurements
Storing solar energy in chemical bonds through the reversible isomerization of UV−vis absorbing molecules offers a promising approach to energy storage. These molecules form high-energy photoisomers, which can store energy if kinetically protected by a significant activation barrier against spontaneous thermal back-isomerization. In this study, we compare the back-isomerization kinetic parameters (ΔH‡ and ΔS‡) of model azobenzene-based photoswitches in solution with those obtained in the gas phase using an original tandem ion mobility mass spectrometer. Our findings show that the activation enthalpy is well-reproduced from the solution phase to the gas phase, whereas the activation entropy is significantly affected by the absence of solvent, revealing further different relaxation mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical Chemistry
化学-分析化学
CiteScore
12.10
自引率
12.20%
发文量
1949
审稿时长
1.4 months
期刊介绍:
Analytical Chemistry, a peer-reviewed research journal, focuses on disseminating new and original knowledge across all branches of analytical chemistry. Fundamental articles may explore general principles of chemical measurement science and need not directly address existing or potential analytical methodology. They can be entirely theoretical or report experimental results. Contributions may cover various phases of analytical operations, including sampling, bioanalysis, electrochemistry, mass spectrometry, microscale and nanoscale systems, environmental analysis, separations, spectroscopy, chemical reactions and selectivity, instrumentation, imaging, surface analysis, and data processing. Papers discussing known analytical methods should present a significant, original application of the method, a notable improvement, or results on an important analyte.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


