三唑类农药在水稻体内的生物积累和转化:定量构效关系、代谢途径和毒性评价
IF 6.2
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
摘要
根吸收是农药进入植物的主要途径;然而,它们的吸收机制和生物转化行为尚不清楚。通过水培试验,研究了21种三唑类杀菌剂在水稻植株上的根吸收和生物转化行为。水稻根系对三唑的吸收可能主要来源于根表面的吸附,而不是营养液的直接支配。QSAR模型显示,与logow和分子极化率相关的分子描述符(如ALogP和ATSC6p)与三唑类杀菌剂的生物积累密切相关。共鉴定出6种典型三唑类农药的26种代谢物,其中6种为首次鉴定。生态结构活性关系预测模型(ECOSAR)软件的毒性预测表明,生物转化产物(如甲基化产物)可能表现出更高的毒性。因此,农药生物转化产品的生态风险值得关注,需要进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
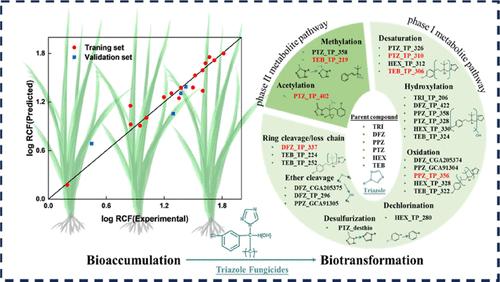
Bioaccumulation and Biotransformation of Triazole Pesticides in Rice (Oryza sativa L.): Quantitative Structure–Activity Relationship, Metabolic Pathways, and Toxicity Assessment
Root uptake is a primary pathway for pesticides to enter the plants; however, their uptake mechanisms and biotransformation behaviors are still lacking. Herein, the root uptake and biotransformation behaviors of 21 triazole fungicides in rice plants (Oryza sativa L.) by hydroponic experiments were investigated. The uptake of triazoles in rice roots may directly and mainly originate from adsorption in the root surface, not directly dominated by nutrient solution. A QSAR model revealed that the molecular descriptors related to log Kow and molecular polarizability (e.g., ALogP and ATSC6p) were closely associated with the bioaccumulation of triazole fungicides. Twenty-six metabolites of six typical triazole pesticides were identified, and six among them were determined for the first time. The toxicity prediction by Ecological Structure Activity Relationships Predictive Model (ECOSAR) software indicated that biotransformation products (e.g., methylation products) may exhibit a higher toxicity. Therefore, the ecological risk posed by pesticide biotransformation products should be a concern, which needs further investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


